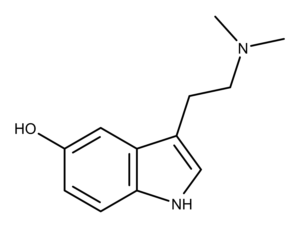
Bufotenin is a toxin discovered in the secretions of the toad Bufo vulgaris.1 Also, bufotenin is found in other organisms like frogs, plants, mammals, and mushrooms.
Although the compounds have similar names, bufotenin should not be confused with bufotenidine, another toxin.
The Chemistry of Bufotenin
The chemical structure of bufotenin was established in 1934.2 In 1935, Hoshino and Shimodaria were the first to synthesize it in the lab.3
The chemical structure of bufotenin is similar to the psychedelic mushroom (aka magic mushroom) compounds psilocin and psilocybin. Bufotenin is psilocin with the hydroxy group at the 5-position instead of the 4-position.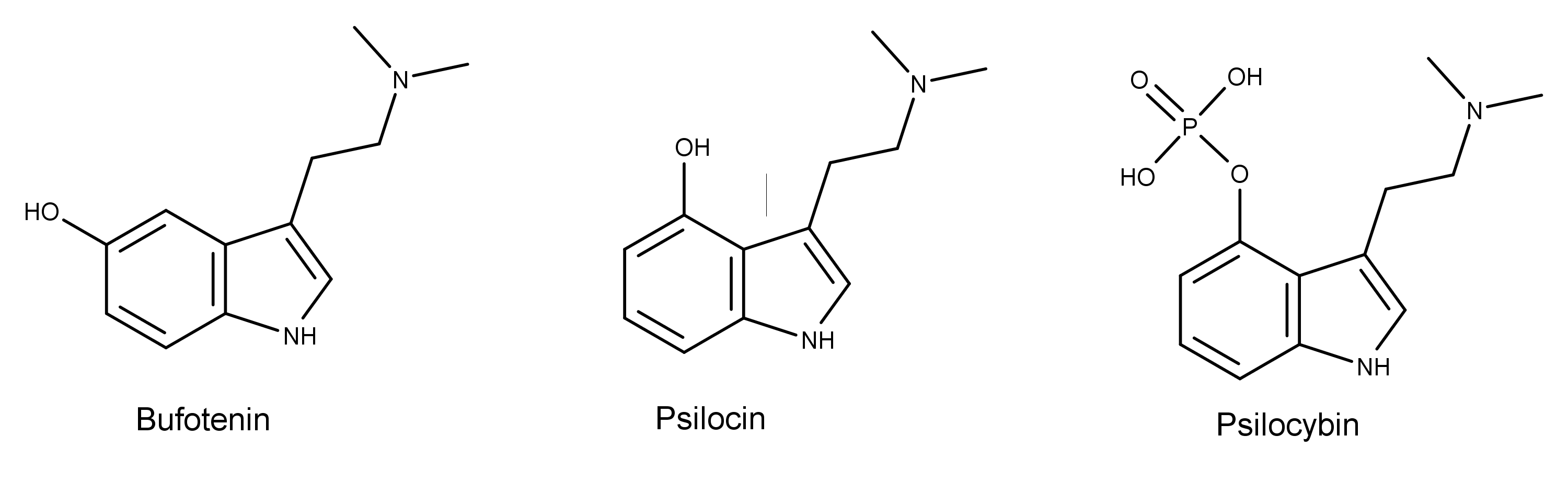
The Pharmacology of Bufotenin
The serotonin receptors are the main site of action for bufotenin and bufotenidine. In particular, their psychoactive properties are due to their potent agonist activity at the 5-HT2A receptor.4 Further, studies indicate that both compounds bind to other serotonin receptors including 5-HT1A and 5-HT1B,5 and 5-HT3.6
A 2017 radioligand binding study found that bufotenine from the parotid gland of the toad Bufo bufo had a greater affinity for neuronal α7 nicotinic acetylcholine receptors compared with muscular cholinergic receptors.7 This is an interesting finding because the α7 receptor is believed to be involved in long term memory function in the brain.
The Applications and Potential of Bufotenin
Overall, when it comes to toad secretions, 5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine) is receiving the majority of research attention right now. However, because of its structural and pharmacological similarity to psilocin and psilocybin, bufotenin presents some intriguing research questions. For example, unlike the relationship between the phosphorylated prodrug psilocybin and its bioactive analog psilocin, a phosphorylated version of bufotenin is not known. Does this mean there is there a yet-to-be-discovered phosphorylated prodrug version that is metabolized to bufotenin?
A 2014 study indicated that bufotenine inhibits the rabies virus infection in in vitro.8 A recent dosing study in mice conducted by the Butantan Institute in Brazil found that an effective dose of bufotenin did not significantly affect the physiology or central nervous system.9 Therefore, the authors suggest bufotenin may be a drug prototype for use in rabies treatment.
Studies indicate that people suffering from schizophrenia and autism often have detectable levels of bufotenin in their brains, urine, and plasma.10–12