MDMA, also known as 3,4-methylenedioxymethamphetamine, is a synthetic recreational psychoactive drug first developed by Merck in 1912.1 The original name Merck gave MDMA was methylsafrylamin. MDMA started becoming available to the public and appearing on the streets in the US in the 1970s in Illinois2,3 and Indiana.4
Some sources report that Merck initially synthesized MDMA as part of their research and development efforts to create an appetite suppressant. However, a 2006 review of Merck’s internal documents disproves this assumption.5 The authors of the review concluded that Merck created and patented MDMA in 1912 as a precursor for a new hemostatic substance (to stop bleeding). The company never tested the pharmacological effects of MDMA at the time. Merck patented MDMA to keep from infringing on an existing patent for a hemostatic substance that had been filed by a German competitor.
The Chemistry of MDMA
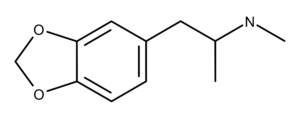
The chemical structure of MDMA is similar to methamphetamine (Figure 1).
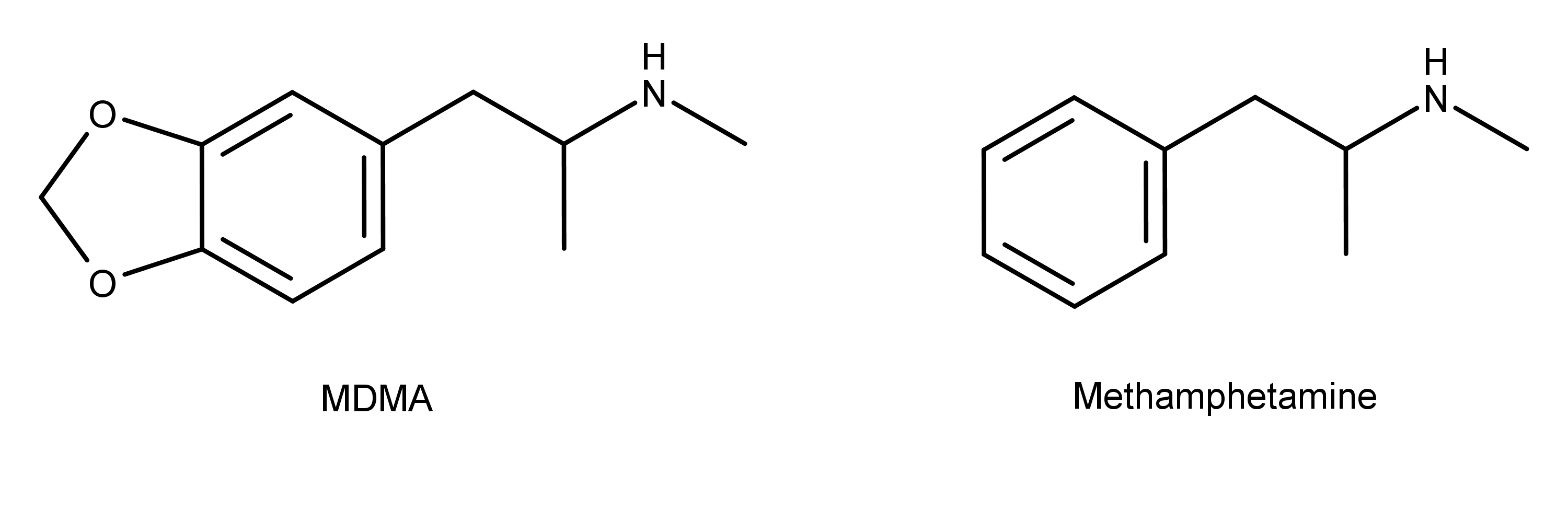
Figure 1: The chemical structures of MDMA and methamphetamine. MDMA has a methylenedioxy group, and methamphetamine does not.
The synthesis of MDMA begins with compounds that contain a preformed methylendioxyphenyl ring such as piperonal, isosafrole, safrole, and piperonylacetone.6 The original synthesis method used by Merck was brominating safrole to 1-(3,4-methylenedioxyphenyl)-2-bromopropane which was then reacted with methylamine.1,6
Safrole is produced primarily from the sassafras plant found mostly in China, Myanmar, and Cambodia.7 Isosafrole is an isomer of safrole, and piperonal is produced by the oxidation of isosafrole. Piperonal also occurs naturally in several plants, including violets, dill, vanilla, and black pepper.
The illicit manufacturing of MDMA often begins with the precursor 3,4-methylenedioxyphenyl-2-propanone (3,4-MDP-2-P).7 The 3,4-MDP-2-P can be synthesized from safrole, isosafrole, or piperonal.
The Pharmacology of MDMA
About 80% of ingested MDMA is metabolized in the liver, and about 20% is excreted in the urine.8,9 In the body, MDMA can be N-demethylated to form 3,4-methylenedioxyamphetamine (MDA), then O-demethylenated to form 3,4-dihydroxymethamphetamine (HHMA). HHMA is further metabolized by O-methylation to form 4-hydroxy-3-methoxy-methamphetamine (HMMA). The majority of MDMA metabolites (especially HMMA which is highly unstable) form conjugates with sulfate glucuronic acid before excretion.10,11
MDMA binds to the three synaptic monoamine transporters serotonin, dopamine, and norepinephrine.12,13 It acts as a releasing agent of these neurotransmitters via its activity at trace amine-associated receptor 1 (TAAR1) and vesicular monoamine transporter 2 (VMAT2).14,15 When MDMA is active at TAAR1, the neurotransmitters move out of the cellular cytosol into the synaptic cleft. MDMA activity at VMAT2 causes the neurotransmitter to move from the synaptic cleft back into the cytosol.
Of the three transporters discussed above, MDMA has the greatest affinity for the serotonin transporter.12 Its affinities for dopamine and norepinephrine be at least 10-fold less. Binding at the serotonin and dopamine transporters is stereoselective with the S-(+) isomer being more potent. No stereoselectivity for the other transporters has been noted.
MDMA has a binding affinity for several classic neurotransmitter receptors, particularly serotonin 5-HT2, α2-adrenergic, muscarinic M1, and histamine H1.13 The compound also binds but has less affinity for M2 muscarinic, α1-adrenergic, ß-adrenergic, 5-HT1, and dopamine D1 and D2 receptors.
The Applications and Potential of MDMA
MDMA-assisted psychotherapy was going on in the US prior to the Drug Enforcement Agency placing it on Schedule I in 1985.16 At the time it concluded, this work was showing that MDMA decreased pain and feelings of fear and anxiety in people while allowing them to remain clear-headed and alert. Research on the therapeutic effects of MDMA has come a long way since then, to the point of clinical trials that are currently underway.
The Multidisciplinary Association for Psychedelic Studies (MAPS) is in the process of spending nearly $27 million dedicated to the pursuit of US Food and Drug Administration (FDA) approval of MDMA as a prescription medicine by 2021. Their first randomized controlled pilot study conducted in 2011 found that “MDMA-assisted psychotherapy can be administered to post-traumatic stress disorder patients without evidence of harm, and it may be useful in patients refractory to other treatments.”17 Currently, MAPS is funding phase 3 clinical trials to evaluate MDMA-assisted psychotherapy for post-traumatic stress disorder (PTSD).18 The results of the trial so far have been so promising that in August 2017, the FDA granted breakthrough therapy designation for MDMA for the treatment of PTSD.
There is evidence suggesting that the effects of MDMA in women are different from men. The 2016 US Global Drug Survey found that female British clubbers were 2-3 times more likely to seek emergency treatment than men after using MDMA (ecstasy).19 There was also a 4-fold increase in the last three years in emergency room visits for women who had used MDMA. Researchers theorize that the cause may be related to women’s unique body chemistry.
A 2001 study found the psychoactive effects of MDMA in women were more intense than those of men, possibly due to women being more susceptible to the serotonin-releasing effects of MDMA.20 The effects reported included perceptual changes, thought disturbances, and the fear of loss of body control. The dose of MDMA was positively correlated with the intensity of the effects. Women also had more adverse effects and outcomes from MDMA than men.
Receptor Binding Affinity Data
| Receptor | Ki (nM) | Species | Note | Ref. |
|---|---|---|---|---|
| 5-HT1A | >10000 | Human | 23 | |
| 5-HT1B | >10000 | Human | 23 | |
| 5-HT1D | >10000 | Human | 23 | |
| 5-HT1E | >10000 | Human | 23 | |
| 5-HT2A | >10000 | Rat | 23 | |
| 5-HT2B | 500 | Human | 23 | |
| 5-HT2C | >10000 | Rat | 23 | |
| 5-HT3 | >10000 | Human | 23 | |
| 5-HT5A | >10000 | Human | 24 | |
| 5-HT6 | >10000 | Human | 23 | |
| 5-HT7 | >10000 | Human | 23 | |
| D1 | >10000 | Human | 23 | |
| D2 | >10000 | Human | 23 | |
| D3 | >10000 | Human | 23 | |
| D4 | >10000 | Human | 23 | |
| D5 | >10000 | Human | 23 | |
| M1 | >10000 | Human | 23 | |
| M2 | >10000 | Human | 23 | |
| M3 | 1851 | Human | 23 | |
| M4 | 8245 | Human | 23 | |
| M5 | 6339 | Human | 23 |