Mescaline is well known for being a compound found primarily in the tops (aka buttons) of the peyote cactus Lophophora williamsii.1 Archaeologists have found evidence of the use of peyote by Native Americans as far back as 5,700 years ago, making it the oldest known plant drug containing a bioactive compound.2
In their book PiHKAL: A Chemical Love Story, Alexander and Ann Shulgin dubbed mescaline one of the “Magical Half Dozen” compounds that they considered the most important out of all those they synthesized and studied.3
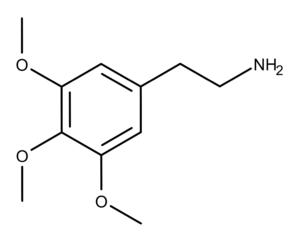
The Chemistry of Mescaline
Mescaline is a phenethylamine alkaloid that is substituted at positions 3, 4, and 5 by methoxy groups.4 It was first isolated by Heffter in 1898.5 Ernst Spath was the first to synthesize mescaline in 1918.6 Slotta and Heller determined its correct molecular structure in 1930.7 The Shulgins described the appearance of pure mescaline synthesized in the lab as “magnificent”, adding, “Long, glistening needles that are, in a sense, its signature and its mark of purity.”3
Over the years, a variety of synthesis methods for mescaline have been developed (reviewed in Cassels and Sáez-Briones, 2018).1 The biosynthesis pathway of mescaline in L. williamsii and other species was determined in the 1960s.8,9 The synthesis begins with the amino acid tyrosine, which is hydroxylated to L-DOPA. Then, L-DOPA is decarboxylated to dopamine, which undergoes hydroxylation to 3,4,5-trihydroxy- ß-phenethylamine (5-hydroxydopamine). In the last step, 3,4,5-trihydroxy- ß-phenylethylamine is methylated to form mescaline. A recent study found genes in L. williamsii that code for enzymes such as tyrosine/DOPA decarboxylase, hydroxylases, and O-methyltransferases which may catalyze some steps of the synethesis.1
The Pharmacology of Mescaline
Pure mescaline, either synthetic or isolated from a natural source, requires relatively high doses to achieve its full psychedelic effects.1 Peyote buttons contain a variety of compounds besides mescaline such as hordenine, N-methylmescaline, pellotine, anhalonine, and lophophorine.10 By themselves, some of these compounds have no psychopharmacological activity. But when administered in certain combinations, they potentiate and alter the effects of mescaline.
Mescaline is an agonist of the serotonin 5-HT2A and 5-HT2C receptors.11 It has a Ki (dissociation constant) of about 550 nM at 5-HT2A and 300 nM at 5-HT2C using cloned human receptors.
Although they are chemically unrelated, the hallucinogenic effects of mescaline are similar to LSD.12 Furthermore, cross-tolerance between mescaline and LSD is known to occur in humans.13
Mescaline has a half-life in the human body of about 6 hours after oral administration.14 The compound is almost completely eliminated (92%) within the first 48 hours, most of it unchanged (55-60%). Urinary excretion peaks at about the second hour after ingestion. Urinary metabolites include 3,4,5-trimethoxyphenylacetic acid (27-30%), N-acetyl-ß-(3,4-dimethoxy-5-hydroxyphenyl) ethylamine (5%), and N-acetyl-mescaline (less than 0.1%). A more detailed description of the metabolism of mescaline is found in Castagnoli, 1978.15
The Applications and Potential of Mescaline
Despite the current interest in psychedelic research, there is virtually no work being done with pure mescaline.1 Historically, research studies have focused on the effects of peyote, which contains a cocktail of chemicals besides mescaline.
However, in 1966, a research team including James Fadiman and Myron Stolaroff studied the effects of mescaline on 27 healthy men, testing for changes in their problem-solving abilities.16 The results indicated that mescaline (and LSD, which they also tested) facilitated created problem-solving in the volunteers. They found the psychedelics particularly effective in the illumination (aka eureka) phase of problem-solving. The data also suggested that the improvement in problem-solving may last for several weeks.