Both the plant and animal worlds are sources of the psychoactive tryptamine compound 5-MeO-DMT. It was first described in plants in a member of the family Rutaceae called Dictyoloma incanescens.1 It is also found in high concentrations in the parotid gland secretions of the Colorado River toad Bufo alvarius.2 Historically, synthetic, toad- and plant-based sources of 5-MeO-DMT have been used for recreational and spiritual purposes.
A 2010 review article cites several references indicating 5-MeO-DMT has been detected in human urine, blood, and cerebrospinal fluid and may also be synthesized in the retina and pineal gland.2 Furthermore, elevated levels of 5-MeO-DMT and its analogs have been detected in the body of fluids of people suffering from psychiatric disorders such as schizophrenia.
The Chemistry of 5-MeO-DMT
5-MeO-DMT is a tryptamine alkaloid that is substituted by a methoxy group at position 5. It was first synthesized by Hoshino and Shimodaira in 1936.3 5-MeO-DMT is a derivative of DMT (N,N-dimethyltryptamine), and the neurotransmitter serotonin (Figure 1).
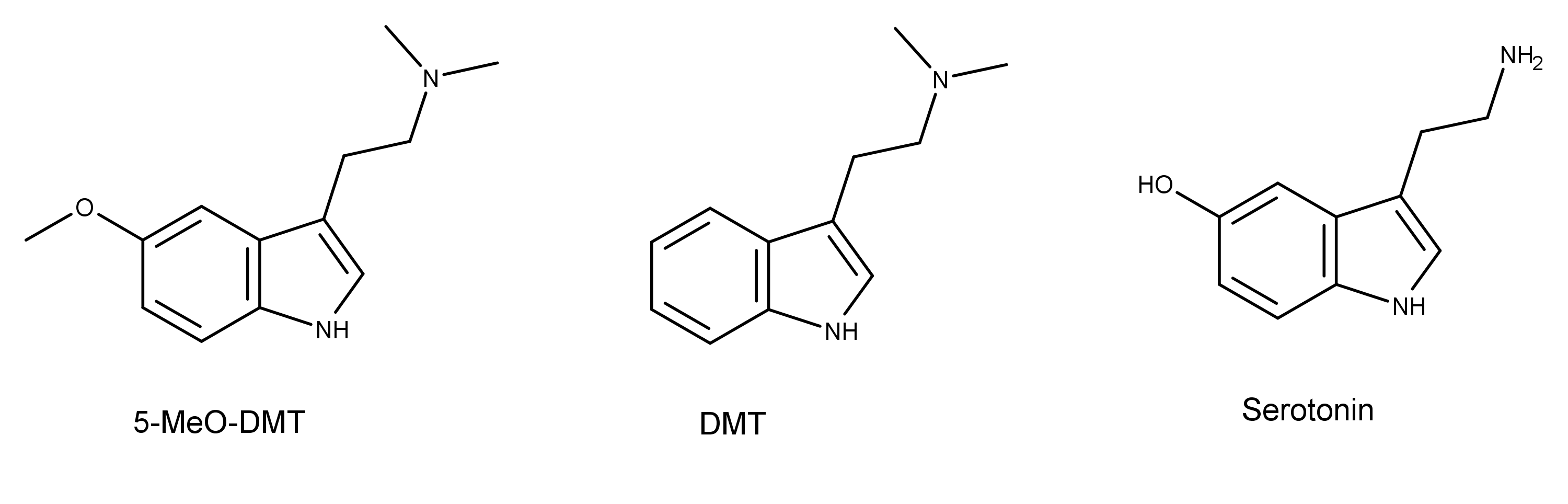
Figure 1: The chemical structures of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), N,N,-dimethyltryptamine (DMT), and 5-hydroxytryptamine (serotonin).
The Pharmacology of 5-MeO-DMT
The psychedelic effects of oral ingestion of 5-MeO-DMT are fast-acting, short-lived. This is because it is quickly rendered inactive due to deamination by the enzyme monoamine oxidase A (MAO-A). 5-MeO-DMT also undergoes O-demethylation by the cytochrome P450 2D6 enzyme.4,5 The O-demethylation of 5-MeO-DMT creates the compound 5-HO-DMT (bufotenine). Studies have shown that 5-HO-DMT has a greater affinity for the serotonin 5-HT2A receptor than 5-MeO-DMT.6-8 Because of the fast oral metabolism, users often inhale the vapor (insufflation) of 5-MeO-DMT.9
Substantial research indicates that the 5-HT1A receptor is important for the effects of 5-MeO-DMT.6,10-14 There is also some experimental evidence indicating agonism at 5-HT1B may also be involved.15-19
The Applications and Potential of 5-MeO-DMT
In humans, research suggests that 5-MeO-DMT improves symptoms of anxiety and depression.20,21 A 2018 study in mice found that a single dose of 5-MeO-DMT stimulates neurogenesis.22 The authors theorize that this effect may help explain the observed antidepressant properties of DMT derivatives. An in vitro study done in 2014 indicates that DMT and 5-MeO-DMT may regulate inflammation and immune homeostasis via the sigma-1 receptor.23
Two recent human studies found beneficial effects from the inhalation of vaporized toad secretions containing 5-MeO-DMT.20,24 However, due to the presence of other compounds in the secretions (the entourage effect), it is difficult to evaluate the effects of 5-MeO-DMT alone.