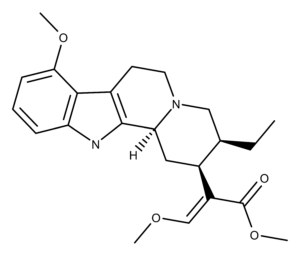
Mitragynine is the primary indole alkaloid compound found in the plant genera Mitragynia, particularly Mitragynia speciosa.1
The Chemistry of Mitragynine
Hooper was the first to isolate mitragynine in 1907.2 Its crystal structure was determined in 1965.3
Studies exploring the total and partial synthesis of mitragynine reveal some interesting findings regarding the structure-activity relationship (SAR) of its molecular scaffold and interaction with the mu opioid receptor (MOR).4–9 For example, the MOR activity of mitragynine stops when small molecular changes are made to the acrylate and ethyl groups on one of the rings. Also, the demethylation of the aryl methoxy group reduces the potency of mitragynine at the MOR.
The Pharmacology of Mitragynine
Studies have been done to examine how mitragynine interacts with the human mu opioid receptor (hMOR).6 Combining docking study observations with what is known about the SAR of mitragynine at MOR, suggests that mitragynine and its structural analogs adopt a distinct binding pose in the receptor pocket. Additional experiments showed that mitragynine had a Ki of 233 nM and was a partial agonist at hMOR.
In addition, studies have shown that mitragynine is active at the human kappa (hKOR) and delta opioid (hDOR) receptors receptors.6 It acts as a competitive antagonist at these receptors. However, the data indicate that mitragynine is only a weak antagonist at hDOR.
Mitragynine also binds to several other receptor types, including dopamine D2, 5-HT2C, and 5-HT7.10
The Applications and Potential of Mitragynine
Studies indicate that mitragynine elicits analgesic effects in several species.11–15 Further, the analgesic effects of kratom may depend on the route of administration. The potency of mitragynine was similar to codeine in several species when it was administered orally or intraperitoneally.15 However, it did not have an analgesic effect in mice and rats when administered subcutaneously.
To explain this phenomenon, the authors of the study suggested that mitragynine was converted to an active metabolite during first-pass metabolism in the body after being given orally or intraperitoneally. Thus, perhaps the analgesic effects were due to a metabolite of mitragynine and not mitragynine itself. However, another study found that mitragynine elicited its analgesic effects when administered directly into the cerebrospinal fluid in the brain, which would eliminate most of the effects (if any) of first-pass metabolism.12
Studies indicate that raw kratom extract has analgesic effects in rodents.13,14,16–18 Also, these studies indicate that mitragynine alone can cause analgesic effects in several species.