The Chemistry of ALD-52
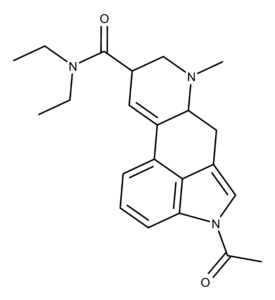
ALD-52 was first synthesized by Franz Troxler and Albert Hofmann in 1957.1 It belongs to the ergoline family of compounds which includes LSD. The chemical structure of ALD-52 differs from LSD by having an acetyl group on the nitrogen atom in the indole ring.
The Pharmacology of ALD-52
Research conducted decades ago found that ALD-52 was psychoactive in humans.2–5 Additional work at the time showed its potency about equivalent to that of LSD.6
A recent study determined Ki values for ALD-57 of 1,054 nM, 174 nM, and 10.2 nM, at serotonin 5-HT1A, 5-HT2A, and 5-HT2C, respectively.7 Receptor activity studies indicated that ALD-52 is a very weak partial agonist at the human 5-HT2A receptor compared to LSD, but it did induce the head-twitch response in mice. However, ALD-52 showed no agonist activity at 5-HT2A, and 5-HT2C despite showing comparatively high affinity those receptors compared to LSD.
At the time Alexander and Ann Shulgin published PiHKAL: A Chemical Love Story, they made an interesting prediction about ALD-52 possibly being a prodrug of LSD:8
If ALD-52 hydrolyses so easily to LSD, and the body is indeed a hydrolytic instrument, then these two drugs should be absolutely equivalent in every particular. This is the ergot equivalent of the psilocybin to psilocin argument, except this is an acetamide rather than a phosphate ester.
The data from the November 2019 study indicates that ALD-52 is indeed a prodrug of LSD.7 High levels of LSD were found in the blood plasma of rats after they were administered ALD-52 subcutaneously.
The Applications and Potential of ALD-52
As a prodrug of LSD, ALD-52 may provide drug formulation options due to its different solubility and other physical properties.