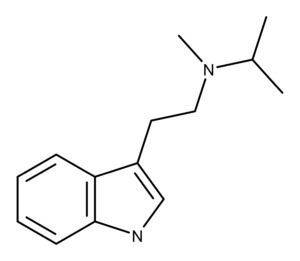
The Chemistry of MiPT
MiPT is the N-methyl-N-isopropyl derivative of DMT (dimethyltryptamine).1 In 1981, Repke et al. reported the synthesis of MiPT.2
In 2019, researchers solved the crystal structure of the fumarate salt of MiPT.3 They describe it as “a protonated tryptammonium cation and a 3-carboxyacrylate (hydrogen fumarate) anion in the asymmetric unit.”
The Pharmacology of MiPT
In 1990, McKenna et al. reported that MiPT was more potent at the serotonin 5-HT1A, 5-HT2A, and 5-HT2B receptors than DMT.4
In their book TiHKAL (see entry #47), Alexander and Ann Shulgin proposed a theory for why orally-administered DMT is not psychoactive while MiPT is active.5 For DMT, monoamine oxidase (MAO) enzymes quickly destroy the methyl group on the terminal nitrogen, rendering the molecule inactive. For MiPT, the Shulgin’s proposes that the large isopropyl group keeps the enzyme away from the nitrogen atom. By blocking the enzyme or its function in some way, they say that it is “…the N-small-group that does the job in the brain.”
The Applications and Potential of MiPT
Other than what is presented herein, there is little known about the pharmacology of MiPT. Solving the crystal structures of the fumarate salt of MiPT presents a new chemistry of this compound for further research to understand its therapeutic potential.