Bolstered by the promising results seen so far, research into the therapeutic effects of psychedelic drugs is continuing to gather momentum. Clinical trials investigating the effects of psilocybin on depression, for example, have been so successful that they were given breakthrough therapy designation in 2018 by the US Food and Drug Administration (FDA). The psilocybin used in these studies is a synthetic, pure form of the compound that is difficult and expensive to manufacture.1 These challenges in making psilocybin have prompted scientists to explore the potential therapeutic effects of derivative of other psychedelics. One of the first steps in this process is solving the crystal structure of a compound.
Building on their work with psychedelic compounds from earlier this year,2,3 researchers have published the crystal structures of the fumarate salts of the N-isopropyl-N-methyl derivatives of DMT and psilocin.4 These derivatives of DMT and psilocin are more commonly known as MiPT and 4-HO-MiPT, respectively. The descriptive names of the two salts are:
- N-isopropyl-N-methyltryptaminium (MiPT) fumarate
- 4-hydroxy-N-isopropyl-N-methyltryptaminium (4-HO-MiPT) fumarate monohydrate
Their systematic names are:
- [2-(1H-indol-3-yl)ethyl](methyl)propan-2-ylazanium 3-carboxyprop-2-enoate, and
- [2-(4-hydroxy-1Hindol-3-yl)ethyl](methyl)propan-2-ylazanium 3-carboxyprop-2-enoate monohydrate
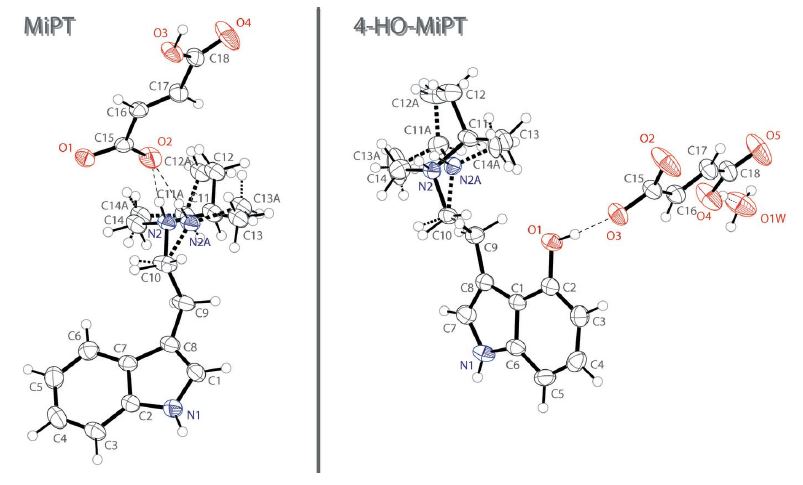
The researchers describe the fumarate salts (Figure 1) as follows:
Both salts possess a protonated tryptammonium cation and a 3-carboxyacrylate (hydrogen fumarate) anion in the asymmetric unit; the 4-HO-MiPT structure also contains a water molecule of crystallization.
Why Are the Fumarate Salts of MiPT and 4-HO-MiPT Important?
In 1990, researchers studied the serotonin receptor affinity of N-methyl-N-isopropyl derivatives of DMT and psilocin.5 They discovered that these derivatives were more potent at 5-HT1A, 5-HT2A, and 5-HT2B receptors compared to analogous dimethyl compounds. Of particular interest is the activity of the compounds at the 5-HT2A receptor which is responsible for the psychedelic effect. The potency of MiPT and 4-HO-MiPT at 5-HT2A makes them candidates for development as psychedelic drugs. And, the fumarate salts of these compounds present new chemistries for further research to understand their therapeutic potential.
Summary
Small changes at the molecular level can translate into significant changes in effect when it comes to drugs. Therefore, working with compounds at the molecular level is essential for unraveling the mysteries of how drugs work and how other compounds affect them. The new crystalline fumarate salts of MiPT and 4-HO-MiPT elucidated by these researchers could be used to modulate the effects of each compound in a drug formulation. This modulation is known in the world of medical cannabis as the entourage effect. In addition, determining the crystal structures of compounds is critical for understanding their physical properties and for probing their activity at receptors (e.g., via modeling studies).