Andrew R. Chadeayne,a* James A. Golenb and David R. Mankeb
aCaaMTech, LLC, 58 East Sunset Way, suite 209, Issaquah, WA 98027
bDepartment of Chemistry and Biochemistry, University of Massachusetts Dartmouth, 285 Old Westport Road, North Dartmouth, MA 02747
*e-mail: Andrew@Caam.Tech
Reviewed by Dr. Glenn P. A. Yap
Introduction
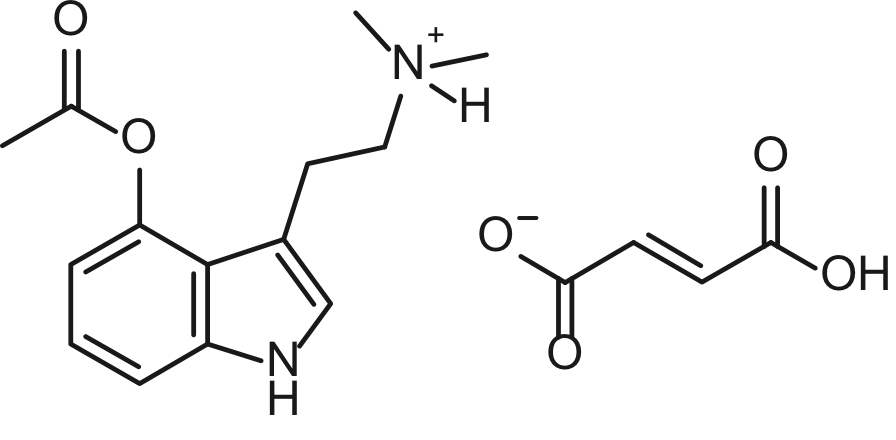
There has been a recent increase in the study of psychedelic agents, as potential pharmaceuticals for the treatment of mood disorders. 4-acetoxy-N,N-dimethyltryptamine, commonly 4-AcO-DMT or psilacetin, is the O-acetyl prodrug of psilocin. Psilocin is the primary metabolite of psilocybin, and the active component of hallucinogenic or “magic” mushrooms. Psilacetin also generates psilocin upon metabolism, but is easier to synthesize than psilocybin. The synthesis of psilacetin was first reported by Nichols in 1999, and the compound is usually produced as the crystalline fumarate salt. Herein, we report the solid state structure of 4-acetoxy-N,N-dimethyltryptamine fumarate.
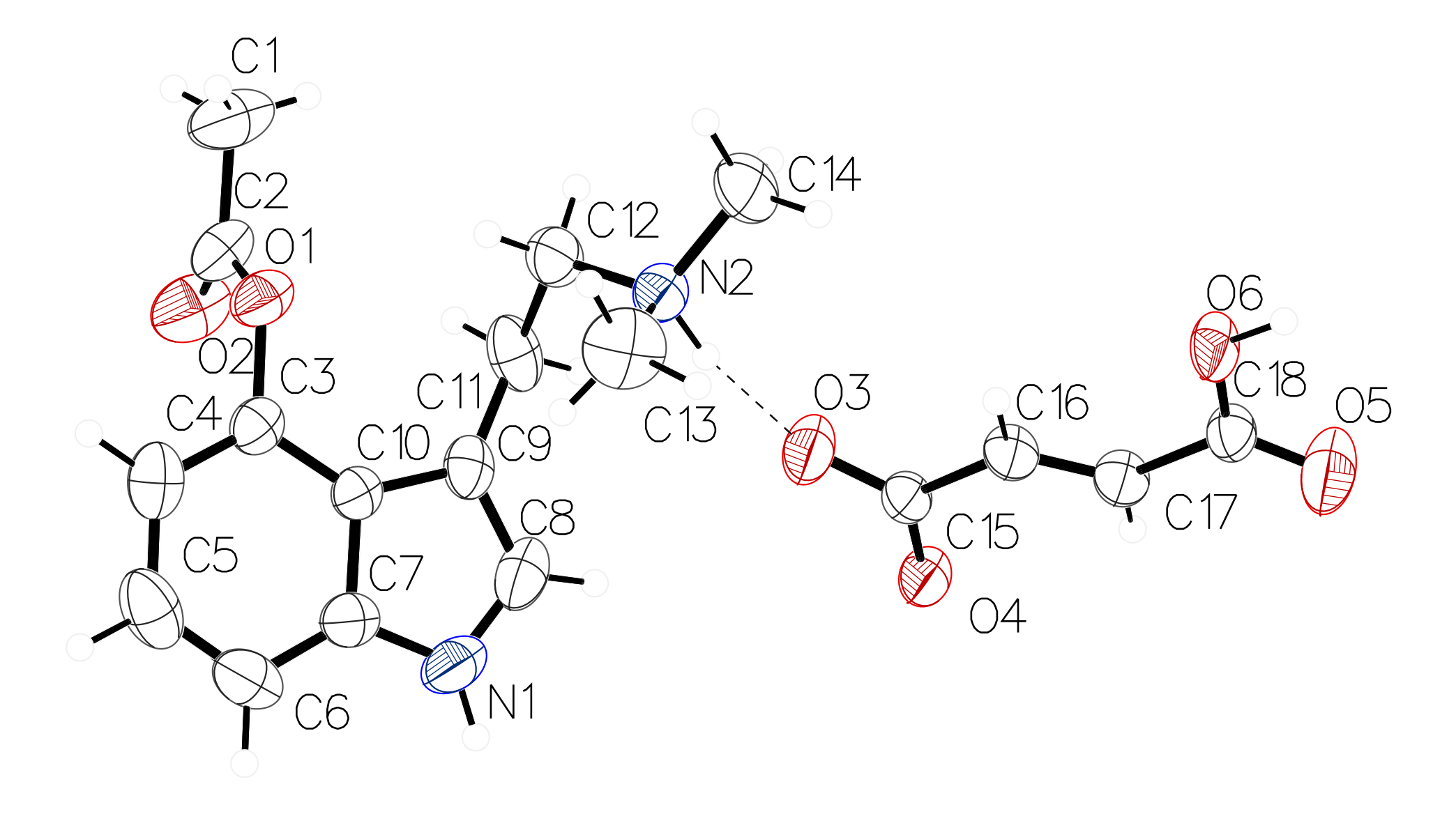
Figure 1. The molecular structure of 4-acetoxy-N,N-dimethyltryptamine fumarate, showing the atom labelling. Displacement ellipsoids are drawn at the 50% probability level. Hydrogen bonds are shown as dashed lines (see Table 1).
Table 1. Hydrogen-bond geometry for 4-AcO-DMT fumarate
| D–H···A | D–H (Å) | H···A (Å) | D···A (Å) | D–H···A (°) |
|---|---|---|---|---|
| N1–H1···O4i | 0.86(3) | 2.03(3) | 2.87(1) | 165(3) |
| O6–H6a···O4ii | 0.99(3) | 1.56(3) | 2.55(2) | 178(2) |
| N2–H2···O3 | 0.90(2) | 1.80(2) | 2.69(2) | 168(2) |
Structure Description
The molecular structure of the fumarate salt of psilacetin is shown in Figure 1. The asymmetric unit contains one 4-acetoxy-N,N-dimethyltryptammonium cation and one 3-carboxyacrylate anion. The indole of the cation is near planar with a mean deviation from planarity of 0.011 Å. The acetate unit is positioned in a perpendicular fashion, with the angle between the indole and acetate planes being 92.75(6)°. The singly protonated fumarate is in the trans configuration and is also nearly planar with a mean deviation from planarity of 0.053 Å. The ions are held together in the solid state through a series of hydrogen bonds (vide infra).
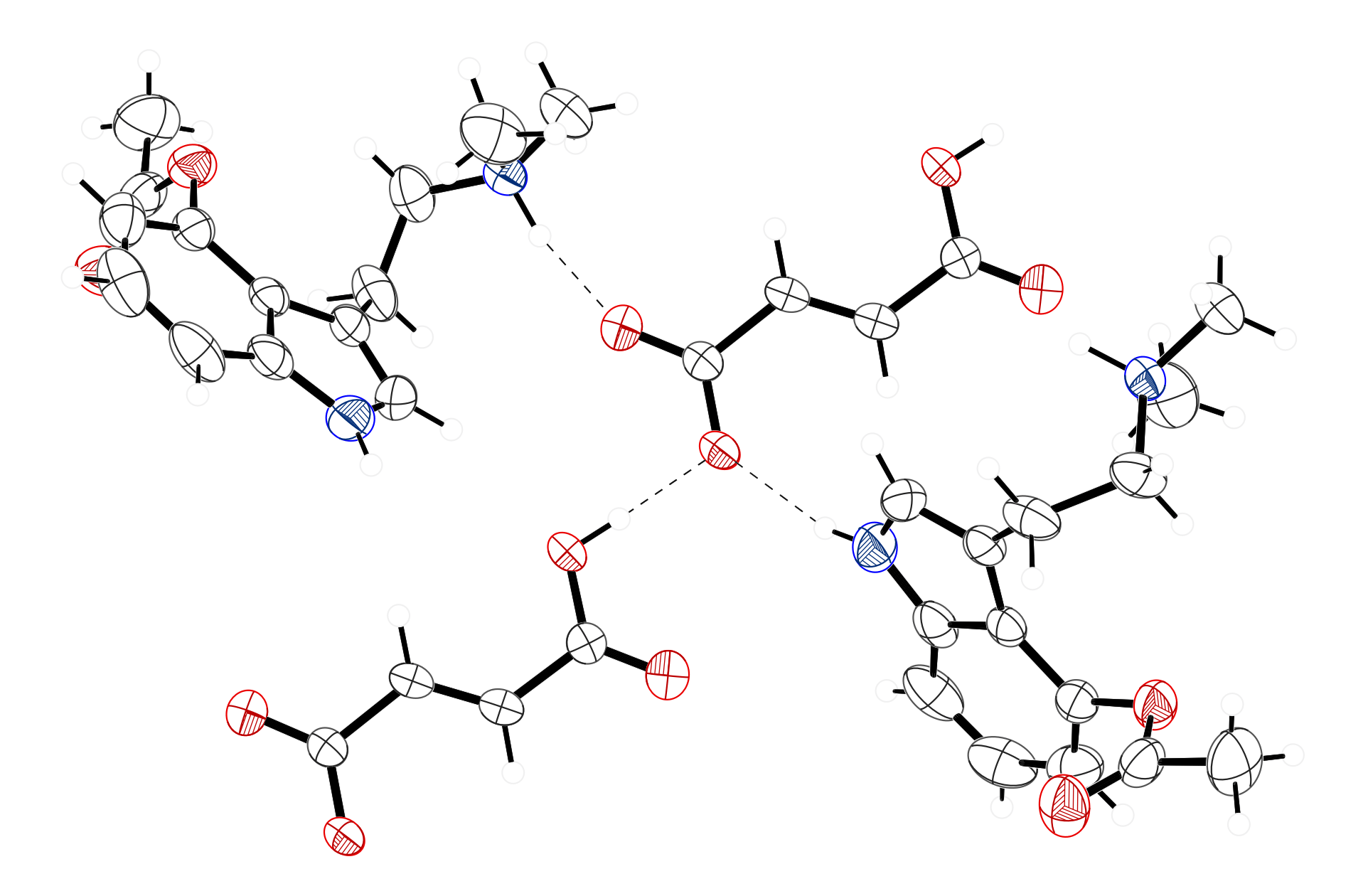
Figure 2. The hydrogen bonding of the fumarate anion in the structure of 4-acetoxy-N,N-dimethyltryptamine fumarate. The acetate end of the fumarate has three hydrogen bonds – one with an ammonium hydrogen, one with an indole hydrogen and one with the carboxylic acid hydrogen of another fumarate.
Supramolecular features
The 4-acetoxy-N,N-dimethyltyptamine and fumarate ions are linked together into infinite chains along the [010] direction through N–H···O and O–H···O hydrogen bonds. One oxygen of the carboxylate on the 3-carboxyacrylate ion forms a hydrogen bond with the proton on the ammonium salt of a psilacetin molecule. The other oxygen of the carboxylate forms a hydrogen bond with an indole hydrogen, and also forms a hydrogen bond with the carboxylic acid of another fumarate ion (Figure 2). The packing of the compound is shown in Figure 3.
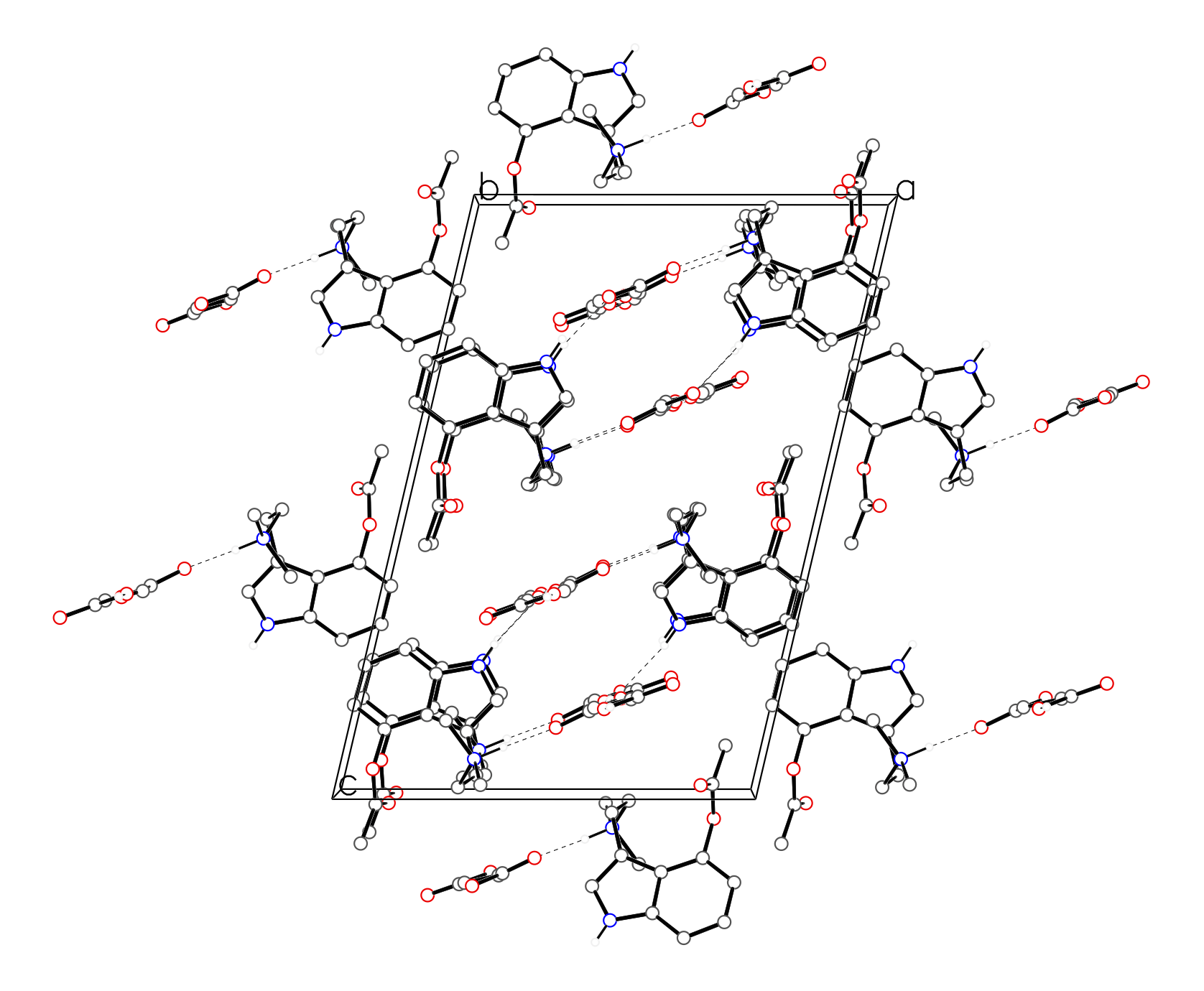
Figure 3. The crystal packing of 4-acetoxy-N,N-dimethyltryptamine fumarate, viewed along the b axis. The N–H···O and O–H···O hydrogen bonds are shown as dashed lines. Hydrogen atoms not involved in H-bonding have been removed for clarity.
Related structures
The two most closely related structures reported are the naturally occurring hallucinogenic agents found in mushrooms: psilocybin and psilocin. The bond distances and angles observed in the dimethyltryptamine units of these two compounds and the structure reported here are nearly identical. In the psilocybin structure one of the phosphate protons is transferred to the dimethylamino nitrogen, producing an ammonium/phosphate zwitterion. The nature of the psilocin structure is not as clear, believed to exist as a statistical mixture of zwitterions and neutral molecules, with a proton involved in an intramolecular hydrogen bond between the hydroxide and the amine. The reported structure differs as it exists as an ion pair between the protonated ammonium salt and the singly deprotonated 3-carboxyacrylate.
Crystallization
A commercial sample (The Indole Shop) of 4-acetoxy-N,N-dimethyltryptamine fumarate was used for crystallization. Colorless crystals were grown by slow evaporation of an aqueous solution.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2. The structure solution was obtained by intrinsic phasing. All non-hydrogen atoms were refined anisotropically (SHELXL) by full-matrix least squares on F2. Hydrogen atoms H1, H2 and H6a were found from Fourier difference maps and refined isotropically with 1.50 Ueq of parent N or O atoms. All other hydrogen atoms were placed in calculated positions with appropriate carbon-hydrogen bond lengths and isotopic parameters: C–H(Ar) 0.930 Å, CH2 0.970 Å and CH3 0.960 Å and 1.20, 1.20 and 1.50 Ueq of parent C atoms, respectively.
Table 2. Crystal data and structure refinement for 4-AcO-DMT fumarate
| Empirical formula | C18H22N2O6 |
| Formula weight | 362.37 |
| Temperature (K) | 296 |
| Crystal system, space group | monoclinic, P21/c |
| a, b, c (Å) | 13.023(1), 7.4823(6), 19.102(2) |
| α, β, γ (°) | 90, 103.251(3), 90 |
| V (Å3) | 1811.8(3) |
| Z | 4 |
| Radiation type | Mo Kα (λ = 0.71073) |
| μ (mm-1) | 0.1 |
| F(000) | 768 |
| Crystal size (mm3) | 0.27 × 0.22 × 0.20 |
| Diffractometer | Bruker D8 Venture CMOS |
| Absorption correction | Multi-scan |
| Reflections collected | 43099 |
| Independent reflections | 3320 [Rint = 0.0366, Rsigma = 0.0144] |
| Data/restraints/parameters | 3320/0/251 |
| Goodness-of-fit on F2 | 1.037 |
| Final R indexes [ I ≥ 2σ (I)] | R1 = 0.0419, wR2 = 0.1024 |
| Final R indexes [all data] | R1 = 0.0522, wR2 = 0.1118 |
| Largest diff. peak/hole (e Å–3) | 0.34/–0.24 |
Acknowledgements
We acknowledge support from the National Science Foundation (award No. CHE-1429086) that purchased the X-ray diffractometer.
Supplementary Material
CCDC 1897284 contains the supplementary crystallographic data for the compound. The data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retreiving/html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Downloads
| Full Paper | The Crystal Structure of 4-AcO-DMT Fumarate | PDF (1 MB) |
| Supporting Information | Supporting Information - The Crystal Structure of 4-AcO-DMT Fumarate | PDF (265 KB) |
| CIF File | UMD1651e_a.cif | Crystallographic Information File (CIF) (265 KB) |