When it comes to the compounds in psilocybin mushrooms (aka magic mushrooms), psilocybin is usually the center of attention. However, there are additional compounds found in magic mushrooms that probably play a role in the overall psychedelic experience for the user.
Baeocystin is a psilocybin derivative or analog. It was first isolated from Psilocybe baeocystis by Leung and Paul in 1968.1 Later, other researchers isolated it from other species such as Psilocybe semilanceata, Panaeolus renenosus, Panaeolus subbalteatus2 and Pluteus salicinus.3
In 1994, Gartz found levels of baeocystin as high as 0.34% dry weight in some Psilocybe species.4 This was about one-third the concentration of psilocybin. Levels of baeocystin this high mean that the compound may be physiologically relevant to the overall effects of the mushrooms.
The Chemistry of Baeocystin
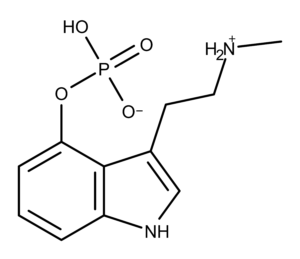
Baeocystin was first prepared synthetically in the lab by Troxler, Sailor, and Albert Hofmann in 1959.5 Chemically, baeocystin differs from psilocybin by one methyl group (Figure 1). Baeocystin is [3-(2-methylaminoethyl)-1H-indol-4-yl] dihydrogen phosphate whereas psilocybin is [3-(2-dimethylaminoethyl)-1H-indol-4-yl] dihydrogen phosphate. Baeocystin is often described as either the N-demethylated derivative of psilocybin or the phosphorylated derivative of 4-HO-NMT (4-hydroxy-N-methyltryptamine).
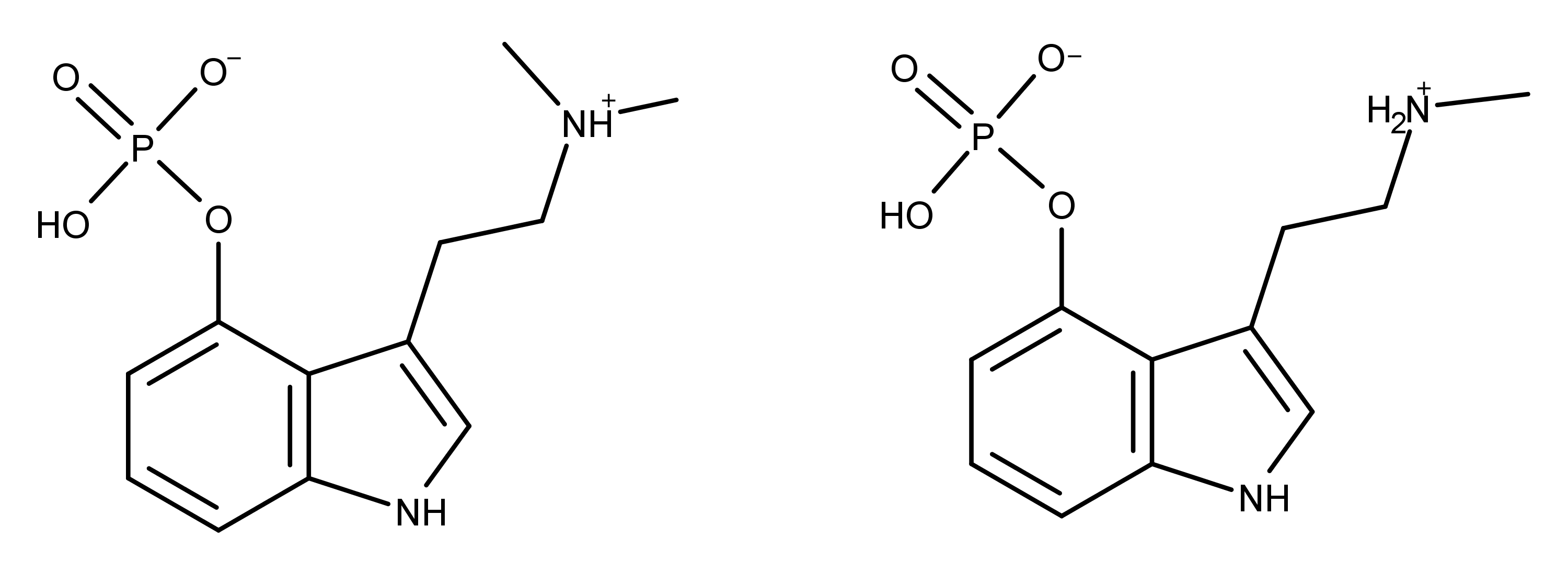
Figure 1: The chemical structures of psilocybin and baeocystin.
Although the presence or absence of a methyl group seems like a small structural change, those sorts of differences often result in significant changes in pharmacology. For example, amphetamine and methamphetamine differ only by one methyl group on their respective ethylamino groups. That difference results in dramatically different activity between the two molecules.
In some cases, the presence or absence of alkyl groups on the amino nitrogen affects the metabolism of the molecule. For example, adding a methyl group to amphetamine (to make methamphetamine) changes how the molecule is metabolized by monoamine oxidase enzymes (MAOs).6
An Important Breakthrough for Researchers
In 2020, Sherwood et al. published a synthetic method for producing baeocystin and other minor magic mushroom compounds in the lab.7 Until this work, no one had useful and meaningful access to these compounds. Below is a high-level overview of their method:
- Synthesis of acyl chloride from 4-acetyoxyindole and oxalyl chloride.
- Acyl chloride is reacted with N-benzylmethylamine or dibenzylamine to yield the desired ketoamides.
- Reduction of the ketoamides using lithium aluminum hydride in tetrahydrofuran (THF) and 2-methyltetrahydrofuran.
- Removal of the ß-hydroxy intermediate (a reactive impurity that is prone to forming dimers) via filtration through a silica pad.
- Phosphorylation using sodium hydride, THF, and ortho-xylenyl phosphoryl chloride (o-XPCl).
- Baeocystin was obtained by catalytic hydrogenolysis using either palladium on carbon or a palladium hydroxide catalyst. This step was followed by filtration, solvent removal, and precipitation by adjusting the pH to afford solid (and in some cases crystalline) products in good yield.
The authors note a key improvement in their method compared to previous work, which eliminates the need to purify the compounds by using chromatography. Their method isolates the compounds by precipitating them from a pH-adjusted aqueous solution via the addition of acetone.
The Pharmacology of Baeocystin
Until 2020, the scientific community had little data on baeocystin or its human pharmacology. The accepted knowledge over the years has been that baeocystin is, as Paul Stamets describes, a “deadly poisonous toxin.”
Previously, there are anecdotal reports from people taking the drug. For example, in the book Magic Mushrooms Around the World, author Jochen Gartz refers to a report that describes “10 mg of baeocystin were found to be about as psychoactive as a similar amount of psilocybin.” 8 Gartz also reported that his experience taking 4 mg of the pure drug caused “a gentle hallucinogenic experience.”
Psychedelic mushroom expert Paul Stamets recently described a similar lack of psychoactive activity upon ingesting pure baeocystin. In an interview with Joe Rogan, Stamets says he took 10 mg of baeocystin but did not feel high. He said,
I was ready for liftoff. I was hoping for liftoff, I know what liftoff feels like, and I didn’t get it.
Although there are scattered instances of people like Gartz and Stamets obtaining pure baeocystin, access to this compound has been extremely rare. Accordingly, progress towards understanding the properties of pure baeocystin has been at a standstill, due in part to the lack of availability of the compound.
The First Biological Testing of Baeocystin and Norpsilocin
In their landmark 2020 paper, Sherwood et al. stated,
Of the known tryptamines found in mushrooms, baeocystin and its putative dephosphorylated metabolite norpsilocin would be the most likely candidates to induce psychedelic-like effects similar to psilocybin and its analogous metabolite psilocin.
To investigate this, the researchers performed two biological activity tests comparing baeocystin to psilocybin.7 The tests were the head twitch response (HTR) in mice and the Gq-mediated calcium flux at 5-HT2A, which measures receptor activation.
The data showed that psilocybin induced HTR in a dose-dependent manner. However, baeocystin was indistinguishable from the control mice that were given saline. The authors concluded that,
…baeocystin alone would likely not induce 5-HT2A receptor-mediated psychoactive effects in vivo.
They proposed that the reason the norpsilocin metabolite did not induce HTR in the mice was due to the action of MAOs. MAOs use oxygen atoms to remove amine groups from molecules. They noted that secondary amines tend to be broken down readily by MAOs compared to tertiary amines.6 Therefore, they hypothesized that norpsilocin (secondary amine) gets degraded faster in the body compared to psilocin (tertiary amine).
The researchers hypothesized that the reason baeocystin did not induce the HTR was that it couldn’t cross the blood-brain barrier (BBB). So, they tested baeocystin’s metabolite norpsilocin using the calcium flux test to see if it activated the serotonin 5-HT2A receptor.
The calcium flux test data indicated that norpsilocin was almost a full agonist and more potent at the human and mouse 5-HT2A receptor compared to psilocin. The Emax for norpsilocin at the human 5-HT2AR was 93% of serotonin compared to psilocin whose Emax was 73%. For the mouse receptor, Emax for norpsilocin was 99% of serotonin compared to psilocin which had an Emax of 72%.
Additionally, the EC50 values (i.e., the potency) for norpsilocin and psilocin at the human 5-HT2AR were 8.4 nM and 4.3 nM, respectively. In this test, the lower the EC50, the greater the potency. Although norpsilocin’s EC50 was greater than that of psilocin, the difference (~ 4.0 nM) is considered negligible. Thus, the authors described the compounds as approximately equipotent. For the mouse 5-HT2AR, the EC50 = 19.0 nM for norpsilocin and 9.9 nM, for psilocin. The authors stated,
Norpsilocin was thus as potent if not more efficacious compared to psilocin…
However, norpsilocin would not be able to elicit its effects at 5-HT2A if it is rapidly broken down by MAOs.
The Applications and Potential of Baeocystin
The work by Sherwood et al. provides data on the first biological screenings of baeocystin and norpsilocin. These data serve as a springboard for other researchers to delve deeper into the chemistry and pharmacology of these compounds. Also, scientists now have a synthesis route for making baeocystin and norpsilocin in the lab. They can make the compounds themselves for study, alleviating the supply problem.
Prior to the 2020 study by Sherwood et al., virtually nothing was known about baeocystin. Previously, the level of understanding of baeocystin led experts in the field to theorize that it wouldn’t be important to the overall cellular or clinical pharmacology of magic mushrooms. Sherwood et al. summarize their findings in this context by saying,
The in vivo data combined with assessment of the pharmacological liabilities suggest it is unlikely that baeocystin or its putative metabolite norpsilocin contribute significantly to centrally mediated psychedelic effects, likely due to rapid degradation by MAO or inability to cross the blood−brain barrier.
However, they pose an intriguing hypothesis for further investigation:
…baeocystin could potentially exert a synergistic effect with psilocin/psilocybin by competing for MAO, effectively increasing psilocin concentration in the blood.
Based on what is known from studying the compounds in other naturally-occurring organisms such as cannabis, it is conceivable that there is also an entourage effect with magic mushrooms. As suggested by Sherwood et al., baeocystin may be very important for generating and modulating specific psychedelic effects. The current state of the art for baeocystin (and psilocybin) can be improved by isolating each of the individual molecules and studying how they affect cellular receptors (e.g., serotonin) alone and in combination with other molecules.