Aeruginascin is one of several compounds found in a single species of psychedelic mushrooms (aka magic mushrooms) known as Inocybe aeruginascens.1 As of 2020, aeruginascin is seldom mentioned in the scientific literature, psychedelic discussion boards, or even experience reports.
The Chemistry of Aeruginascin
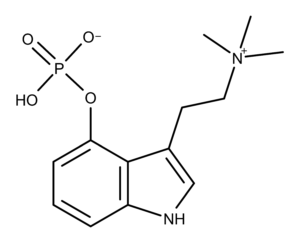
The chemical structure of aeruginascin is similar to psilocybin, the primary psychedelic prodrug in magic mushrooms. Aeruginascin has three methyl groups on the ethanolamine moiety, while psilocybin has two (Figure 1). The additional methyl group creates a positively charged trimethylammonium group on aeruginascin compared to a dimethylamine group on psilocybin. Both of these compounds are structural analogs of the neurotransmitter serotonin, as shown in Figure 1.
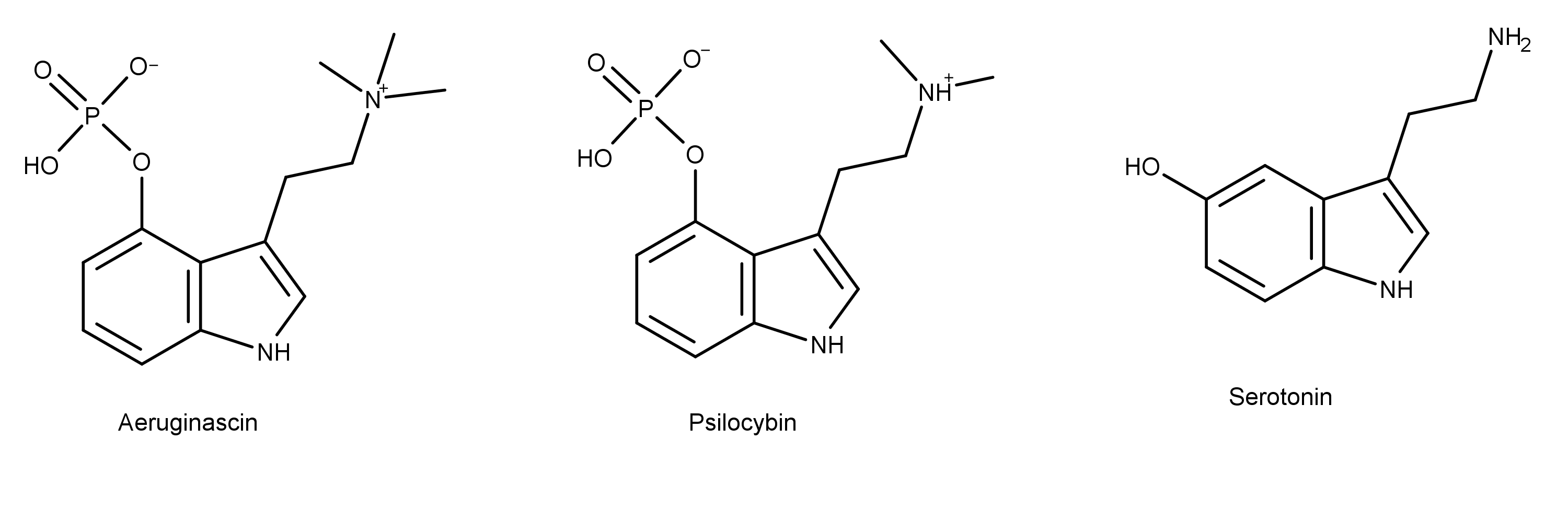
Figure 1: The similar chemical structures of the tryptamines aeruginascin, psilocybin, and serotonin.
The German chemist Jochen Gartz was the first to isolate aeruginascin from extracts of I. aeruginascens.2 He found from further analysis that the concentration of aeruginascin in the mushrooms was comparable to that of psilocybin and baeocystin.3 The quaternary trimethylammonium structure of aeruginascin was confirmed using 1H-NMR in 2004.3
From a chemical structure standpoint (in addition to the compounds shown in Figure 1) aeruginascin is similar to baeocystin and norbaeocystin (Figure 2). It is also closely related to a compound in toad secretions (aka venom) called bufotenidine (Figure 2). Specifically, aeruginascin has three methyl groups on the ethylamine moiety. In contrast, psilocybin has two methyl groups, and baeocystin has just one. Because of the similarity of its chemical structure to bufotenidine, aeruginascin may play a role in the condition known as Wood Lover Paralysis.
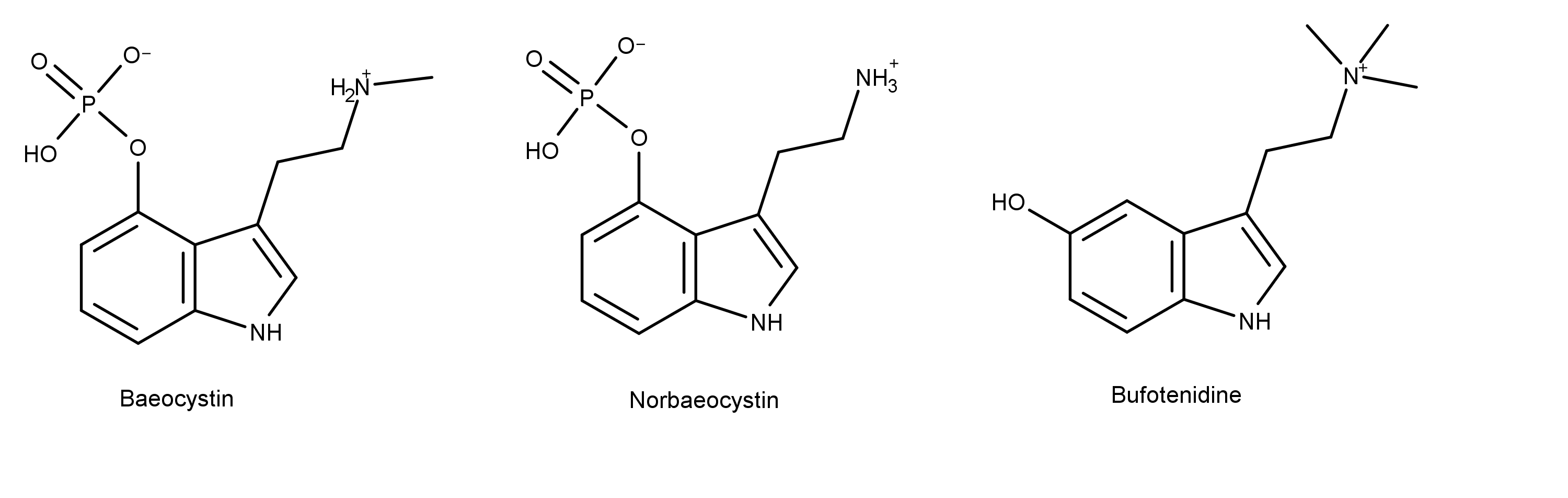
Figure 2: The chemical structures of baeocystin, norbaeocystin, and bufotenidine.
Based on its chemistry, scientists don’t think that aeruginascin can cross the blood-brain barrier.4 Similar compounds entering the human body would have to be demethylated to become metabolically active or be attached to a transporter protein or other molecule.
In 2020, Sherwood et al. published a synthetic method to produce aeruginascin.5 They made aeruginascin by quaternization of psilocybin “using excess methyl iodide in methanol treated with aqueous ammonium hydroxide.” The aeruginascin was collected by filtration and washing with methanol.
The Pharmacology of Aeruginascin
As of 2020, scientists know virtually nothing about the pharmacology of aeruginascin. What it does in the body and how it works is still a mystery.
The only information in the scientific literature about the effects of aeruginascin is from a 1989 study published by Gartz.3 In this work, he analyzed 23 cases of the accidental hallucinogenic mushroom poisonings. He noticed that people who had ingested Inocybe aeruginascens reported only euphoric experiences. Dr. Gartz described the experiences of those ingesting mushrooms without aeruginascin (and high in psilocybin and psilocin) as an “often slight and in some cases deep dysphoric mood” accompanied by psychosis, panic, and anxiety.
Dr. Gartz hypothesized that aeruginascin modified the overall psychedelic effect of the compounds in the mushrooms, culminating in an overall euphoric feeling. Specifically, he stated:
It seems that the significant amounts of the indole derivative aeruginascin can modify the pharmacological action of psilocybin to give an (sic) euphoric mood during psychosis with hallucinations due to ingestion of I. aeruginascens.
As Dr. Gartz observed, the entourage effect is likely at work in the overall psychedelic effects of I. aeruginascens. Understanding and harnessing the pharmacology of aeruginascin may lead to more effective therapies for a variety of conditions, as well as advances for users seeking enhancement of their personal and professional lives.
In their 2020 paper, Sherwood et al. discussed the potential importance of aeruginascin (and/or its active metabolite 4-hydroxy-N,N,N-trimethyltryptamonium aka 4-OH-TMT) in wood lover paralysis (WLP).5 However, the authors did not report any findings regarding the biological activity of aeruginascin or 4-OH-TMT. Further research could help answer questions like whether 4-OH-TMT is involved in so-called bad trips, or if it is the cause of the temporary paralysis seen in WLP. So, as of today, there is still an unmet need for studying the biological activity of aeruginascin.
The Applications and Potential of Aeruginascin
Aeruginascin remains an understudied psilocybin derivative that may have significant contributions to make in psychedelic therapy and personal growth. Unfortunately, there is no research being done on aeruginascin, its chemistry, or its effects in combination with other compounds in magic mushrooms.