Psilocybin is just one of several compounds that naturally occur in psychedelic mushrooms (aka magic mushrooms). One of these other compounds, norbaeocystin, is present at lower concentrations than psilocybin in some mushroom species.1–3 Norbaeocystin is virtually unknown to most people (including many researchers) and is its effects are not well documented.
The Chemistry of Norbaeocystin
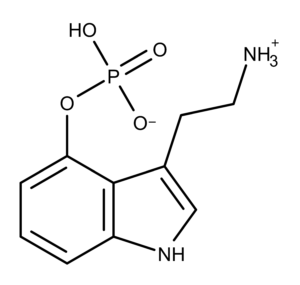
Norbaeocystin is a derivative and analog of psilocybin. It was first isolated from Psilocybe baeocystis by Leung and Paul in 1968.4 In the study, solvent extractions provided material enriched in psilocybin derivatives. Those chemicals were further separated by chromatography to provide pure fractions of norbaeocystin, baeocystin, and psilocybin. Chemically, norbaeocystin is an N-demethylated derivative of baeocystin (and baeocystin is the N-demethylated derivative of psilocybin) (Figure 1).
Recently, researchers demonstrated that norbaeocystin could be an intermediate in the biosynthesis of psilocybin.5 In vitro tests showed that L-tryptophan is converted to tryptamine by the enzyme PsiD which is then converted to 4-hydroxytryptamine by the enzyme PsiH. PsiD can also synthesize 4-hydroxytryptamine directly from the substrate 4-hydroxy-L-tryptophan. Norbaeocystin is then synthesized by the enzyme PsiK. The researchers propose that additional methyl groups are added by the enzyme PsiM, generating baeocystin (one methylation) and psilocybin (two methylations).

Figure 1: The chemical structures of baeocystin, norbaeocystin, and psilocybin.
In 2020, Sherwood et al. published a synthetic method for producing norbaeocystin and other minor magic mushroom compounds in the lab.6 Until this work, no one had useful and meaningful access to these compounds. Below is a high-level overview of their method:
- Synthesis of acyl chloride from 4-acetyoxyindole and oxalyl chloride.
- Acyl chloride is reacted with N-benzylmethylamine or dibenzylamine to yield the desired ketoamides.
- Reduction of the ketoamides using lithium aluminum hydride in tetrahydrofuran (THF) and 2-methyltetrahydrofuran.
- Removal of the ß-hydroxy intermediate (a reactive impurity that is prone to forming dimers) via filtration through a silica pad.
- Phosphorylation using sodium hydride, THF, and ortho-xylenyl phosphoryl chloride (o-XPCl).
- Noraeocystin was obtained by catalytic hydrogenolysis using either palladium on carbon or a palladium hydroxide catalyst. This step was followed by filtration, solvent removal, and precipitation by adjusting the pH to afford solid (and in some cases crystalline) products in good yield.
The authors note a key development in their method compared to previous work, which eliminates the need to purify the compounds by using chromatography. Their method isolates the compounds by precipitating them from a pH-adjusted aqueous solution via the addition of acetone.
The Pharmacology of Norbaeocystin
Norbaeocystin is generally regarded as non-psychedelic. However, there is no evidence to support this belief. Furthermore, the absence of psychedelic properties does not exclude its potential for therapeutic uses — for example, in modulating the properties of drugs and/or endogenous neurotransmitters (e.g. serotonin) — without an intoxicating effect.
Drawing an analogy to the accepted metabolic pathway in mushrooms for the synthesis of psilocybin,5 one could infer that norbaeocystin is dephosphorylated in the body to form 4-hydroxytryptamine, which is further metabolized. Notably, the proposed 4-hydroxytryptamine compound would differ from serotonin (5-hydroxytryptamine) only by the position of the hydroxy- group. Norbaeocystin is hydroxylated at the 4th position in the molecule, whereas serotonin is hydroxylated at the 5th position.
No one has quantified the effects of this molecule on its own or in combination with other active magic mushroom compounds. Although Sherwood et al. presented a much-needed synthesis method for this compound, they did not test its biological properties.6 Accordingly, progress towards understanding the properties of pure norbaeocystin will likely come from better access to the molecule via separation techniques, synthetic methods, or both.
The Applications and Potential of Norbaeocystin
Nothing is known about the possible therapeutic effects of norbaeocystin either by itself or in combination with other magic mushroom compounds. Future research in this area will benefit from shifting the focus from magic mushrooms to the active molecules within them. It is conceivable that norbaeocystin could have therapeutic applications that come without intoxicating properties.
The work done by Sherwood et al. in 2020 presents a huge step forward for studying norbaeocystin. However, they did not present a synthesis method for norbaeocystin’s putative active metabolite 4-hydroxytryptamine. As such, there still remains an unmet need for studying the biological activity of norbaeocystin and its active metabolite.
Based on what is known from studying the compounds in other naturally-occurring organisms such as cannabis, it is conceivable that there is also an entourage effect with magic mushrooms. Norbaeocystin may play an essential role in generating and modulating specific psychedelic effects. The current state of the art for norbaeocystin (and psilocybin) can be improved by isolating each of the individual molecules and studying how they affect cellular receptors (e.g., serotonin) alone and in combination with other molecules.