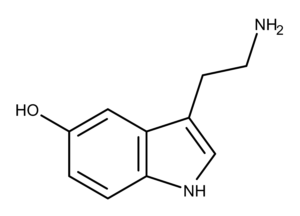
Serotonin is a hormone and monoamine neurotransmitter found in a variety of organisms from single-celled eukaryotes like paramecium to worms, insects, plants, and mammals including humans.1–3
Based on the diverse organisms that express its receptors, scientists think serotonin is one of the oldest signaling molecules in evolutionary history. The primordial forms of the modern-day serotonin receptors are believed to have appeared 700-750 million years ago.4
Serotonin is sometimes called a happiness molecule or confidence molecule.5 Studies have found that alterations in tryptophan (the precursor of serotonin) levels in the body have significant effects on a person’s mood.6,7 For example, low levels of serotonin contribute to depression, while higher levels create a more positive and confident mood.
Using positron emission tomography, researchers found that subjective levels of happiness were positively correlated with increased serotonin synthesis in the right anterior cingulate cortex of human volunteers.8 The same study found that it works the other way around, too. Self-induced changes in a person’s mood influenced serotonin synthesis in the brain.
The Chemistry of Serotonin
Serotonin was known as enteraime when it was first extracted from chromaffin cells of the gut in 1937.9 The first isolation of serotonin was done by Rapport et al., in 1948.10 It’s chemical structure was also elucidated by Rapport in 1949.11
The indoleamine skeleton of the serotonin molecule is also present in psychedelic compounds such as psilocybin, psilocin, norpsilocin, baeocystin, norbaeocystin, aeruginascin, and LSD (lysergic acid diethylamide). It’s not surprising that these psychedelic compounds elicit their effects via the same receptors that serotonin uses (see Pharmacology section below).
In mammals, serotonin is produced from tryptophan via two enzymatic steps. The first step is rate-limiting and consists of hydroxylation of the fifth carbon of the indole ring by tryptophan hydroxylase.12 This creates the compound 5-hydroxytryptophan (5-HTP). The next step is the decarboxylation of the side chain by aromatic amino acid decarboxylase, which produces serotonin.
There are two enzyme isoforms (closely related variants) of tryptophan hydroxylase. The first, now called tph1, was initially isolated from the gut.13,14 The isoform expressed exclusively in the brain is called tph2.15,16
A 2015 study found that indigenous spore-forming microbes in the gut regulate levels of serotonin in the blood and the colon.17 The microbes do this by modulating the metabolites that are needed for serotonin biosynthesis. These results suggest that altering and controlling microorganisms in the colon could help people with serotonin-related conditions or diseases.
The Pharmacology of Serotonin
Shortly after the serotonin was isolated and its chemical structure determined, it was found in the central nervous system of mammals in significant amounts with varying concentrations in different regions of the brain.18,19 This led to the theory that serotonin is a neurotransmitter. At the same time, researchers saw the similarities between the tryptamine moiety present in LSD (lysergic acid diethylamide) and the chemical scaffold in serotonin.
In 1954 paper in the Proceeding of the National Academy of Sciences, researchers Woolley and Shaw wrote of their idea “that the mental disturbances caused by lysergic acid diethylamide were to be attributed to an interference with the action of serotonin in the brain.” 20
Serotonin is present in many body tissues, including the gut, brain, lungs, kidneys, and platelets (reviewed by Mohammad-Zadeh et al., 2008).21 The effects of serotonin are varied and wide-ranging. It plays a role in gastric motility, vasoconstriction, development, endocrine function, mood, sleep, appetite, memory, and learning, to name a few functions. In mammals, most of the serotonin (about 90%) is produced by enterochromaffin cells in the gut. Large quantities of serotonin are stored in blood platelets. The cells of the raphe nuclei are the primary source of serotonin in the brain.22
The receptors unique to serotonin are called serotonergic receptors. They belong to a large receptor family known as the GPCRs (G protein-coupled receptors). There are several subtypes of serotonin receptors that PSR has delved into more deeply in a previous article. The serotonin 5-HT2A receptor is of particular interest to researchers studying the effects of psychedelics. A study done in 2019 on healthy volunteers confirmed that the 5-HT2A receptor is involved in creating the psychedelic experience.23
The Applications and Potential of Serotonin
The SSRI (selective serotonin reuptake inhibitors) class of antidepressants interfere with the normal reuptake of serotonin by neurons.24 This interference causes an increase in the concentration of serotonin in the synaptic cleft. This, in turn, makes more serotonin available at the postsynaptic receptors. This is the basic theory behind how SSRI drugs are thought to work.
Although many decades have passed since the first SSRIs were introduced in the 1980s, scientists still aren’t sure about their exact mechanisms of action. In addition, there continues to be much debate as to the effectiveness of SSRIs.25–27 Adding to the challenges of SSRIs is the myriad of side effects associated with their use continues to make them a less-than-ideal treatment for many patients.28 Also, there remains the underlying issue that SSRIs don’t work for about 30% of people with depression.