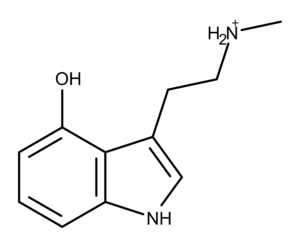
Norpsilocin is a derivative of psilocybin discovered in 2017. Scientists at the Hans-Knöll Institute in Jena, Germany, isolated it from the psychedelic mushroom (aka magic mushroom) Psilocybe cubensis.1 The research team, which included Dr. Dirk Hoffmeister, defined the structure of norpsilocin using 1D and 2D NMR spectroscopy.
The researchers also developed a new extraction method that prevents dephosphorylation of the mushroom chemicals so the true metabolic profile of Psilocybe can be determined. From this, they call norpsilocin a “natural product” of the Psilocybe mushroom and go on to say,
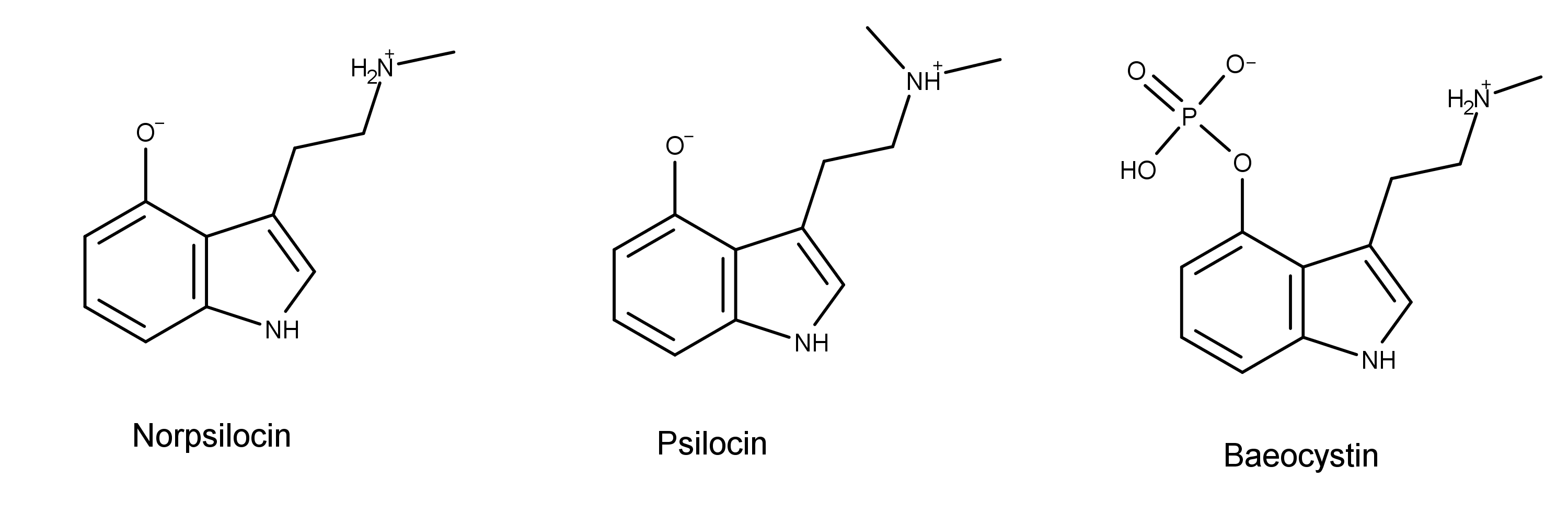
It likely represents the actual psychotropic agent liberated from its 4-phosphate ester derivative, the known natural product baeocystin.
The Chemistry of Norpsilocin
Chemically, norpsilocin is psilocin with one less methyl group on the terminal nitrogen. Norpsilocin is also the presumed dephosphorylated metabolite of another magic mushroom compound, baeocystin.1,2
In 2020, Dr. Alexander Sherwood of the Usona Institute and his research team published a synthetic method for producing norpsilocin and other minor magic mushroom compounds in the lab.2 Until this work, no one had useful and meaningful access to these compounds. Below is a high-level overview of their method:
- Synthesis of acyl chloride from 4-acetyoxyindole and oxalyl chloride.
- Acyl chloride is reacted with N-benzylmethylamine or dibenzylamine to yield the desired ketoamides.
- Reduction of the ketoamides using lithium aluminum hydride in tetrahydrofuran (THF) and 2-methyltetrahydrofuran.
- Removal of the ß-hydroxy intermediate (a reactive impurity that is prone to forming dimers) via filtration through a silica pad.
- Phosphorylation using sodium hydride, THF, and ortho-xylenyl phosphoryl chloride (o-XPCl).
- Norpsilocin was obtained by catalytic hydrogenolysis using either palladium on carbon or a palladium hydroxide catalyst. This step was followed by filtration, solvent removal, and precipitation by adjusting the pH to afford solid (and in some cases crystalline) products in good yield.
The authors note a key improvement in their method compared to previous work, which eliminates the need to purify the compounds by using chromatography. Their method isolates the compounds by precipitating them from a pH-adjusted aqueous solution via the addition of acetone.
Also in 2020, a team of scientists from CaaMTech and the University of Massachusetts Dartmouth reported the synthesis of two new crystalline forms of norpsilocin.3 The April 2020 study published in Acta Crystallographica describes the crystal structures of norpsilocin (4-hydroxy-N-methyltryptamine, 4-HO-NMT) as a free base and a fumarate salt. This paper is the first to describe any salt form of norpsilocin. Andrew Chadeayne, the lead author of the study, posted on LinkedIn: “CaaMTech is happy to announce that we have made and characterized the first pure crystalline forms of norpsilocin….we are grateful for the opportunity to contribute to the fundamental understanding of these understudied compounds. We hope our work supports further research into these interesting compounds.”
The Pharmacology of Norpsilocin
In 2020, Sherwood et al. conducted the first biological testing of norpsilocin.2 As part of the study, the researchers first found that baeocystin did not cause the head twitch response (HTR) in mice. They hypothesized that this was because baeocystin could not cross the blood-brain barrier (BBB). The researchers then thought that if this was true, maybe its metabolite norpsilocin could. If so, it was intriguing that there was no HTR from administering baeocystin.
The authors attributed the lack of activity by norpsilocin in the HTR test to the action of monoamine oxidase enzymes (MAO). MAOs use oxygen atoms to remove amine groups from molecules. They noted that secondary amines tend to be broken down readily by MAOs compared to tertiary amines.4 Therefore, they hypothesized that norpsilocin (secondary amine) gets degraded faster in the body compared to psilocin (tertiary amine).
For the next step in the study, the researchers used a different test to see if norpsilocin directly activated at the 5-HT2A receptor. If the test showed it did, then it would add strength to their theory of it being deactivated by MAOs. To test the ability of norpsilocin to activate the 5-HT2A receptor, they used a test that measures the Gq-mediated calcium flux at 5-HT2A.
The 5-HT2A is present in the membrane of many cell types, including neurons. Activation of 5-HT2A causes cells to release calcium from their inner stores located in the endoplasmic reticulum, which then exits the cell.5,6 Scientists can quantify the activation of 5-HT2A by measuring the calcium flux from the cells.
The calcium flux test data indicated that norpsilocin was almost a full agonist and more potent at the human and mouse 5-HT2A receptor compared to psilocin. The Emax for norpsilocin at the human 5-HT2A was 93% of serotonin compared to psilocin whose Emax was 73%. For the mouse receptor, Emax for norpsilocin was 99% of serotonin compared to psilocin which had an Emax of 72%.
Additionally, the EC50 values (i.e., the potency) for norpsilocin and psilocin at the human 5-HT2A were 8.4 nM and 4.3 nM, respectively. In this test, the lower the EC50, the greater the potency. Although norpsilocin’s EC50 was greater than that of psilocin, the difference (~ 4.0 nM) is considered negligible. Thus, the authors described the compounds as approximately equipotent. For the mouse 5-HT2AR, the EC50 = 19.0 nM for norpsilocin and 9.9 nM, for psilocin.
The Applications and Potential of Norpsilocin
The 2020 work by Sherwood et al. gives researchers the first meaningful access to norpsilocin for scientific study. By solving the crystal structures of norpsilocin and its fumarate salt, CaaMTech is setting the stage for making formulations with precise amounts of psychedelic compounds. Also, the fascinating observation that norpsilocin is more potent than psilocin raises more questions and presents a myriad of research opportunities. Overall, there remains an unmet need for further study of the biological activity of norpsilocin.