
Norpsilocin is one of the newest compounds discovered in magic mushrooms. In 2017, Claudius Lenz et al. isolated it from the carpophores (the stem part of the fruiting body) of Psilocybe cubensis.1 The researchers detected it while they were analyzing extracts from P. cubensis using 1D and 2D NMR spectroscopy.
In the same study, Lenz et al. also developed a new extraction method that prevents the dephosphorylation of the mushroom compounds. This allows the true metabolic profile of Psilocybe to be determined. From this, the authors called norpsilocin a “natural product” of the Psilocybe mushroom. They went on to propose this hypothesis,
It likely represents the actual psychotropic agent liberated from its 4-phosphate ester derivative, the known natural product baeocystin.
Since its discovery, norpsilocin was not studied further until a paper published in February 2020 by Sherwood et al. in the Journal of Natural Products.2 The data from this study appear to corroborate the hypothesis of Lenz et al. that the magic mushroom compound baeocystin is a prodrug of norpsilocin. However, much mystery remains.
Background
Chemically, norpsilocin has one methyl group on the terminal nitrogen, while psilocin has two. Norpsilocin is also the metabolite resulting from the dephosphorylation of baeocystin.
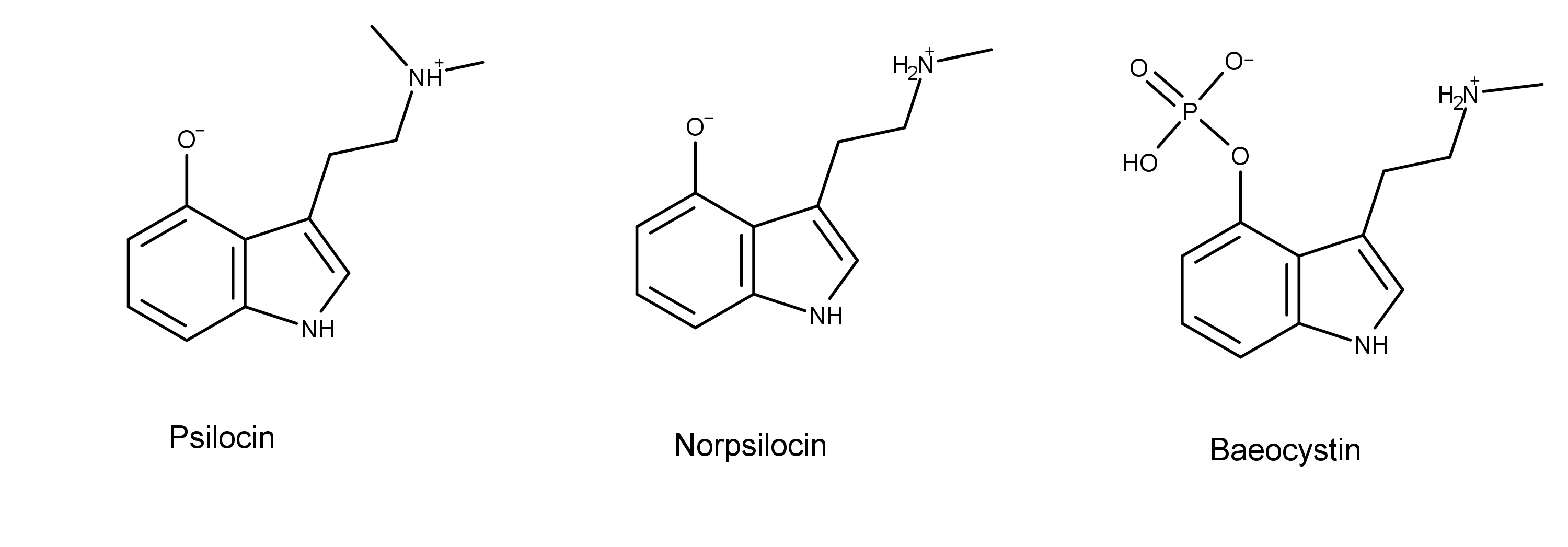
Recall that psilocybin is metabolized to psilocin in the body by removal of psilocybin’s phosphate group.3 Sherwood et al. theorized that, like psilocybin, baeocystin underwent dephosphorylation in the body to for the active compound, norpsilocin.
To test this theory, they evaluated norpsilocin to see if it activated the serotonin 5-HT2A receptor (5-HT2AR) using a test that measures the Gq-mediated calcium flux at 5-HT2A.2
The 5-HT2AR is present in the membrane of many cell types, including neurons. Activation of 5-HT2AR causes cells to release calcium from their inner stores located in the endoplasmic reticulum, which then exits the cell.4,5 Scientists can quantify the activation of 5-HT2AR by measuring the calcium flux from the cells.
Test Results
The calcium flux test data indicated that norpsilocin was almost a full agonist and more potent at the human and mouse 5-HT2A receptor compared to psilocin. The Emax for norpsilocin at the human 5-HT2AR was 93% of serotonin compared to psilocin whose Emax was 73%. For the mouse receptor, Emax for norpsilocin was 99% of serotonin compared to psilocin which had an Emax of 72%.
Additionally, the EC50 values (i.e., the potency) for norpsilocin and psilocin at the human 5-HT2AR were 8.4 nM and 4.3 nM, respectively. In this test, the lower the EC50, the greater the potency. Although norpsilocin’s EC50 was greater than that of psilocin, the difference (~ 4.0 nM) is considered negligible. Thus, the authors described the compounds as approximately equipotent. For the mouse 5-HT2AR, the EC50 = 19.0 nM for norpsilocin and 9.9 nM, for psilocin.
In summation, the authors stated,
Norpsilocin was thus as potent if not more efficacious compared to psilocin in Gq-mediated calcium flux at the 5-HT2A receptor.
The Sherwood et al. study also found that baeocystin did not elicit the head twitch response (HTR) in mice. They theorized that this inactivity might be due to baeocystin not being able to cross the blood-brain barrier. So, the next question became, if inactive baeocystin is metabolized to bioactive norpsilocin in the body, then why doesn’t administering baeocystin cause the HTR in mice?
The authors attribute the lack of activity by norpsilocin in the HTR test to the action of monoamine oxidase enzymes (MAO). MAOs use oxygen atoms to remove amine groups from molecules. They noted that secondary amines tend to be broken down readily by MAOs compared to tertiary amines.6 Therefore, they hypothesized that norpsilocin (secondary amine) gets degraded faster in the body compared to psilocin (tertiary amine).
Moving Forward with Norpsilocin
This landmark study is the first biological testing of norpsilocin in a laboratory setting. Learning that it is more potent at 5-HT2AR than psilocin is an intriguing finding for further investigation. However, what role could norpsilocin play if it is deactivated by MAOs in the body?
Historically, psilocybin and psilocin receive all the attention in psychedelic research. This first of its kind study by Sherwood et al. brings norbaeocystin (and other magic mushroom compounds) into the light. It is essential that all the compounds in magic mushrooms are studied individually as well as in combination. This is because it is feasible that there is an entourage effect with the compounds in magic mushrooms like what is seen with cannabis.

Great article and we need more of this research to go mainstream so that clinical trials don’t end up using chemically derived single compounds in place of the synergistic use of the whole fungi and//or plant body.