
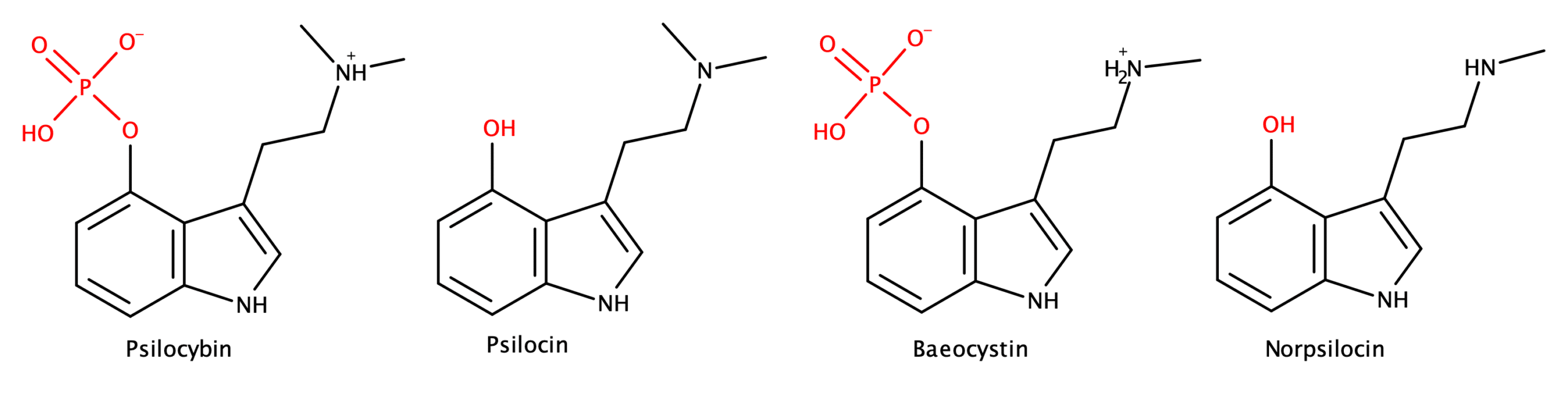
Psilocybin and its active metabolite psilocin are well known components of magic mushrooms (Figure 1). Due to exciting results highlighting the potential of using these compounds as mental health treatments, they have emerged as a topic of significant scientific interest. But, until recently, very little attention had been paid to other close structural analogs of these compounds, which are also present in magic mushrooms.
A recent paper in ACS Chemical Neuroscience by Alexander Sherwood et al. reported that making minor modifications to the chemical structure of the monoalkyl tryptamine norpsilocin (4-hydroxy-N-methyltryptamine) results in a collection of compounds with significant psychedelic activity.1
Norpsilocin Background
In 2017, norpsilocin was first identified in magic mushrooms, i.e. Psilocybe cubensis, by Dirk Hoffmeister’s research group at the Hans-Knöll Institute in Jena, Germany2 (Figure 1).
Norpsilocin is a structural analog of psilocin, differing only by a single methyl group on the nitrogen of the tryptamine’s ethanolamine arm.
In February 2020, a team led by Alexander Sherwood at the Usona Institute published in vivo data for norpsilocin (and it’s naturally occurring prodrug, baeocystin), demonstrating that norpsilocin was “as potent if not more efficacious” than psilocin at the 5-HT2A receptor but paradoxically did not cause a head twitch response (HTR) in mouse models.3 Norpsilocin’s potency at 5-HT2A (the receptor associated with psychedelic effects) would traditionally correlate with observing HTR (the gold standard for predicting a human psychedelic experience) in murine models. So, the lack of observed HTR for a 5-HT2A agonist generated questions for further research.
Shortly thereafter, scientists at CaaMTech synthesized and published the first crystalline forms of norpsilocin, including its freebase and fumarate salt.4 And two years later, the CaaMTech and Usona teams combined forces with the Designer Drug Research Unit (DDRU) of the National Institute on Drug Abuse (NIDA), publishing a paper about the structure activity relationship (SAR) for tryptamines found in magic mushrooms and their synthetic analogs.5 That paper confirmed the Usona team’s earlier findings that norpsilocin did not induce HTR. The findings also eliminated the possibility that the observed lack of HTR for norpsilocin was caused by norpsilocin’s activity at 5-HT1A by showing that co-administering a 5-HT1A antagonist did not uncover HTR. Ultimately, the team, led by Grant Glatfelter, posited that the lack of observed HTR for norpsilocin could be due to poor blood-brain-barrier (BBB) penetration or rapid metabolism of norpsilocin by monoamine oxidase enzymes.
Norpsilocin Derivatives Show Increased Lipophilicity and Robust HTRs
The 2024 Sherwood et al. study was driven by the hypothesis that modifying norpsilocin’s secondary methylamine group would increase lipophilicity and brain permeability, resulting in psychedelic-like effects.1 To test this hypothesis, the researchers synthesized eight norpsilocin derivatives– each modifying the methyl group of norpsilocin to a different group, including primary and secondary alkyl, allyl, and benzylamine groups (Figure 2). The psychedelic-like effects of these analogues were assessed using the HTR assay. They found that extending the N-methyl group to its ethyl and propyl variants notably enhanced the compounds’ cLogP value and presumably their lipophilicity and brain permeability (cLogP, Figure 2).
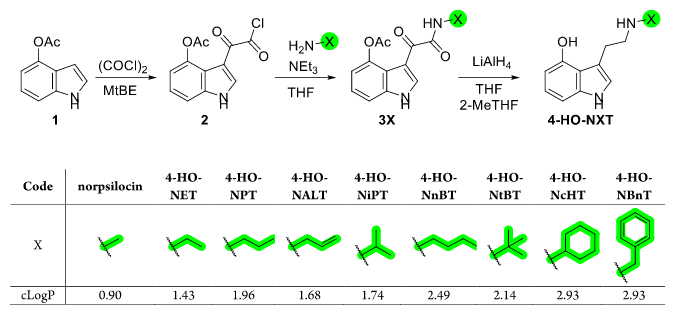
Figure 2: Synthesis of norpsilocin-related analogues with nomenclature and corresponding partition coefficient (cLogP) values calculted using Chemdraw Professional 22.2 (2023, PerkinElmer Informatics).1
Consistent with these findings, the norpsilocin derivatives having increased cLogP values induced robust head-twitch responses (Figure 3) in mice, a hallmark of psychedelic activity. Notably, the 4-HO-NET, 4-HO-NALT, and 4-HO-NBnT exhibited the highest potency with respect to HTR count, significantly surpassing that of norpsilocin.
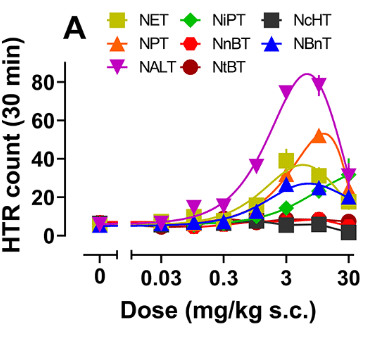
Figure 3: Dose−response and time-course data for the HTR induced by norpsilocin analogues. (A) Dose−response curves for effects on the HTR (n =5−6 mice/dose).1
Pharmacological assays further revealed that these compounds displayed high affinity for the serotonin 5-HT2A receptor, a known target for classic psychedelics. Interestingly, the study found that the length and structure of the alkyl group significantly influenced the psychedelic-like activity and receptor affinity.
For the 5-HT2A receptor subtype, the potency rank order by half-maximal effective concentration (EC50) was 4-HO-NBnT > 4-HO-NALT > norpsilocin >4-HO-NET > 4-HO-NPT > 4-HO-NiPT > 4-HO-NnBT > 4-HO-NcHT > 4-HO-NtBT.
This finding underscores the importance of seemingly small molecular modifications in influencing the pharmacological profile of psychedelic compounds in both in vitro and in vivo assays.
Conclusions
The results from Sherwood et al. 2024 represent a significant contribution to understanding the SARs of psychedelic compounds. By systematically altering the molecular structure of norpsilocin and evaluating the resulting changes in both the compounds’ physical properties (cLogP, lipophilicity) and their biological activity, the researchers have provided valuable insights into how multiple factors contribute to observed properties of a compound. This research not only deepens our understanding of the pharmacology of psychedelics but also provides clues for designing new compounds with particular properties.
This study marks a significant advancement in psychedelic research, offering the first exploration of the SARs of norpsilocin analogues or monoalkyl tryptamines. The findings highlight the nuanced interplay between ligand receptor binding (binding affinity), lipophilicity, and psychedelic activity, highlighting the potential importance of monoalkyl tryptamines – a previously understudied class of molecules.
We would love to hear your thoughts on this story. Feel free to comment below.
If only “murine” communication were possible, we might get an indication about the particular subjective effects of the different modifications, if any.
That the Head Twitch Response is itself a manifestation of actual psychedelic (visual) effects in the mouse is quite the revelation, but should have been obvious all along i.e. how much different could fundamental neuronal function be among mammals?