
Psychedelic research is experiencing many scientific advances for producing psychedelic compounds. This is because trying to get meaningful and usable amounts of psilocybin and psilocin from magic mushroom flesh, for example, is not practical. The levels of these compounds in dried mushrooms are only 0.2% – 1.0% of their dry weight.1 Having ways to make meaningful amounts of these compounds at a reasonable cost is essential for conducting scientific studies from receptor assays to clinical trials in humans.
The Novo Nordisk Foundation Center for Biosustainability in Denmark recently published a study in the journal Metabolic Engineering describing how they bioengineered Saccharomyces cerevisiae to produce psilocybin and other related tryptamine derivatives.2
PSR previously reported on several papers describing both synthetic and bioengineering methods for producing psychedelic compounds and derivatives. Both the new techniques (like the one described herein) and improvements on existing methods are critical for the advancement of psychedelic science. Two of these articles are Scientists Engineer E. Coli to Produce Psilocybin and Scientists Synthesize and Test the Magic Mushroom Compounds Baeocystin, Norbaeocystin, Norpsilocin, and Aeruginascin.
What is S. cerevisiae and Why Use It?
S. cerevisiae is a type of yeast. Dr. Nick Milne et al. chose to work with it “due to its long use in industrial production as well as the fact that it naturally produces very few secondary metabolites or other tryptophan derivates, thereby facilitating simple downstream processing and purification.”
They also chose this yeast species because it naturally expresses cytochrome P450 enzymes that convert tryptamine to 4-hydroxytryptamine (read more about this in the section below, Optimizing the Synthesis of Psilocybin). The presence of the enzyme is a significant cost-savings, according to the authors. It eliminates an extra synthesis step used by other yeasts, which require an expensive substrate during production.
Modifying Yeast to Produce Psilocybin Analogs
The research team inserted genetic material from magic mushrooms into S. cerevisiae. Specifically, they cut out certain psilocybin-making genes from the magic mushroom Psilocybe cubensis and put them into S. cerevisiae. They did this by using plasmids as vectors for transporting the genes into the target yeast cells. Once inside, the plasmid incorporates the new genes into the yeast’s DNA (the plasmids are “programmed” to know where to insert them). The modified yeast then starts making psilocybin according to the instructions encoded in the magic mushroom’s DNA.
The Yeast Also Makes Psilocybin Derivatives
Notably, the researchers also detected several psilocybin derivatives being produced by their bioengineered yeast. The observed psilocybin analogs included psilocin, norpsilocin, baeocystin, norbaeocystin, 4-hydroxytrimethyltryptammonium (i.e., dephosphorylated aeruginascin), and the “new to nature” N-acetyl-4-hydroxytryptamine.
Why was only the dephosphorylated version found and not aeruginascin itself? The researchers noticed that the amount of psilocybin that S. cerevisiae made “was accompanied by a concomitant production of psilocin.” They theorized that the same phosphatase enzymes (of which there are several in S. cerevisiae) acting on psilocybin to produce psilocin were doing the same thing to aeruginascin. The action of these enzymes may also explain the presence of norpsilocin, which is dephosphorylated baeocystin.
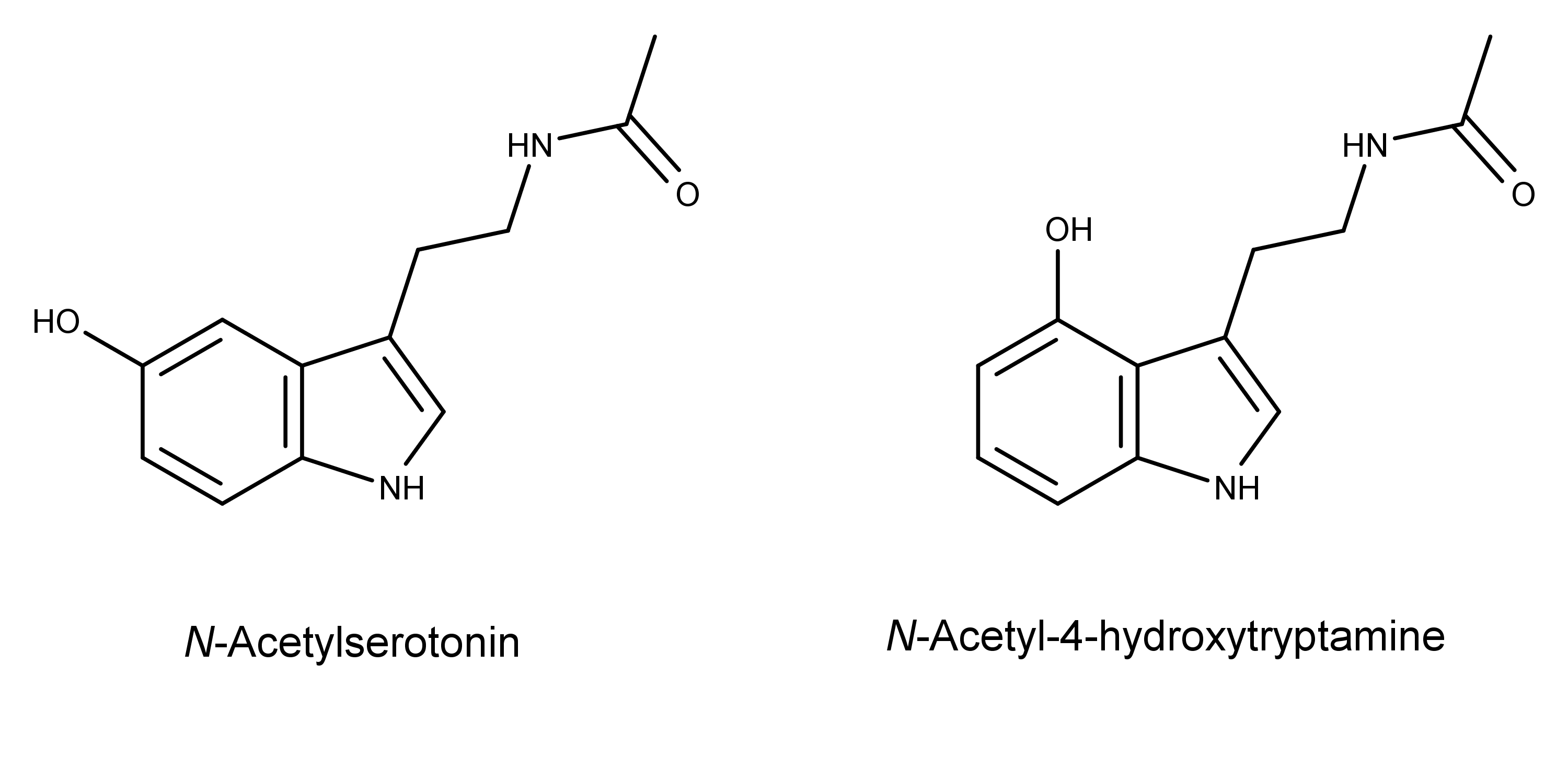
Another result of this study was the creation of a biosynthetic method for producing a “new-to-nature” tryptamine derivative, N-acetyl-4-hydroxytryptamine.
The researchers made the new compound by adding the gene for the enzyme serotonin N-acetyltransferase into the DNA of a different strain of S. cerevisiae that produces 4-hydroxytryptamine. The compound is structurally similar to the neurotransmitter N-acetylserotonin (normelatonin), differing only in the position of the hydroxyl group in the 4-position instead of the 5-position.
According to the authors, this was,
…the successful production of a novel molecule structurally similar to both psilocin and normelatonin with potentially novel pharmacological activity.
Optimizing the Synthesis of Psilocybin
Initially, the amount of psilocybin produced by the genetically engineered S. cerevisiae was low. The researchers noticed that as the yeast was making psilocybin, there was an increase in extracellular tryptamine. The accumulation of tryptamine suggested to them that its conversion to 4-hydroxytryptamine inside the yeast was not very efficient.
The researchers improved the conversion of tryptamine to 4-hydroxytryptamine by giving the yeast a more efficient gene from P. cubensis. The gene, known as cytochrome P450 reductase, catalyzes the conversion of tryptamine to 4-hydroxytryptamine. After this gene was added to the DNA of S. cerevisiae, the data showed a 29-fold increase in the production of psilocybin and psilocin and significantly reduced levels of extracellular tryptamine.
Continuing Research on Magic Mushroom Compounds is Needed
This research provides another method for producing meaningful amounts of psilocybin analogs for scientific study and downstream applications. Also, synthesis of the novel compounds N-acetyl-4-hydroxytryptamine and unphosphorylated aeruginascin by strains of S. cerevisiae provides more opportunities for research into their chemistry and pharmacology.
Taking genes from one organism and splicing them into another are well-known techniques. Nevertheless, the application of this technology to create psychedelic compounds represents an exciting new area of research. Other scientists have recently noted that supply problems have limited research into the “minor” tryptamines in magic mushrooms.3 They also hypothesize that these compounds could have substantial therapeutic value alone or when combined with psilocybin. This recent work discussed above could offer new possibilities for supplying minor tryptamines for further research and development.

Excellent article. Detailed, comprehensive.
mentira tras mentira para producir negocio de un hongo sagrado y para eliminar la sabiduría ancestral y el espíritu del mismo
For purposes of comparison, what is the rate of hydrolysis of psilocybin in water? I’d be curious as to how these hydrolysis reactions (conversion of 4-phosphate to 4-OH) compare to analogous reactions conducted in the absence of phosphatase enzymes. I suspect that pH could have an important influence on the hydrolysis rate. According to the paper, “Fermentation was carried out at 30 °C, with 1 vvm aeration and the pH controlled at 5.0 by addition of a 12% NH4OH solution” and chromatography was conducted using “mobile phase consisting of 20% 10 mM ammonium formate (pH 3) and 80% acetonitrile, with… Read more »
Working hard all week for my money so when the week end comes I go and get phosphorylated