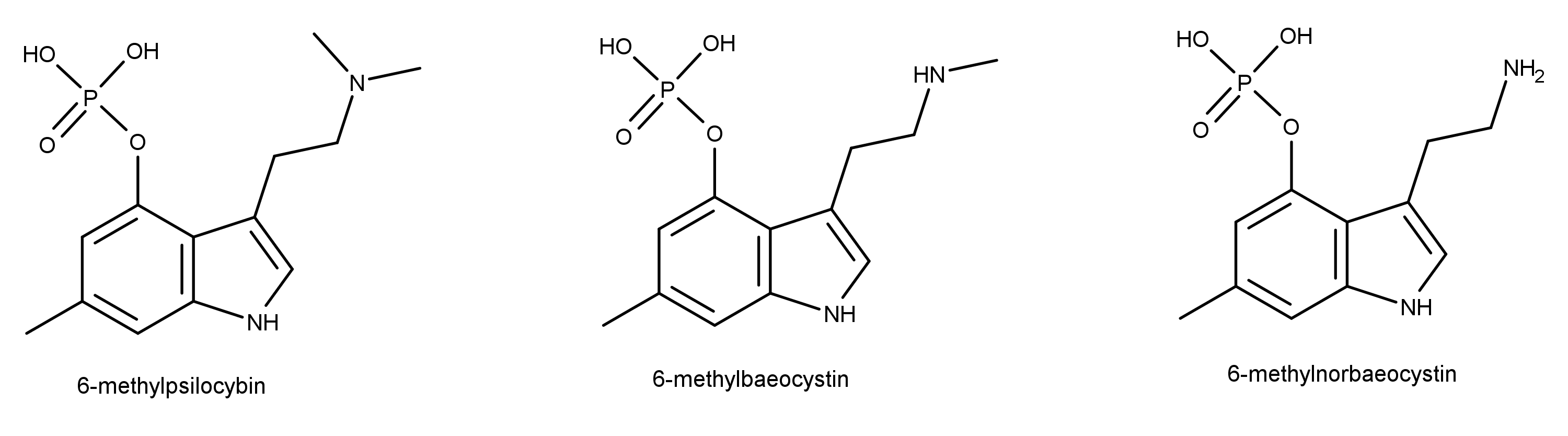
In a May 2019 study, a team comprised of researchers from the United States, Germany, and Austria published their in vitro biosynthesis of 6-methylated psilocybin congeners in the journal ChemBioChem.1 Specifically, they used naturally-occurring enzymes to synthesize the compounds shown in Figure 1, 6-methylpsilocbyin, 6-methylbaeocystin, and 6-methylnorbaeocystin. This study is important because these compounds provide a new way to make psilocybin and they can be used in other studies for a better understanding of how serotonergic psychedelics work.
The route of biosynthesis of the 6-methylated congeners uses some of the same enzymes that Psilocybe mushrooms (aka “magic mushrooms”) use to convert tryptophan to psilocybin such as PsiD, PsiK, and PsiM.2 The synthesis route starts from a compound called 4-hydroxy-6-methyl-L-tryptophan which, as the name says, is already methylated at the 6th carbon. This compound is decarboxylated and phosphorylated using the enzymes PsiD and PsiK from Psilocybe cubensis. Then, the PsiM enzyme is used for methylating the amine group.
The authors cite several studies showing that the methylation of indole compounds at carbon atom 6 “…does not cause consistent pharmacological effects.” This lack of activity is important because these compounds should not interfere with other biochemical pathways if they are going to be part of an effective and useful synthesis route.
Next, the authors of this study plan to work on scaling up the in vitro synthesis of these 6-methylated congeners of psilocybin so they can conduct in vitro assays. One of their main goals is “to discover agonistic or antagonistic effects on 5-HT receptors and, ultimately, for more profound insight into the pharmacology of serotonergic psychedelics.” 1