Bufotenidine is a naturally-occurring toxin found in frog and toad skin and plants.1,2 Although they have similar chemical names, bufotenidine should not be confused with bufotenin, another toad secretion.
The Chemistry of Bufotenidine
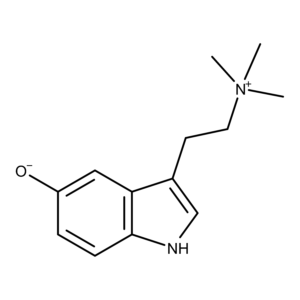
Bufotenidine is the trimethylammonium salt of bufotenin.3 Wieland et al. determined its chemical structure in 1934.4
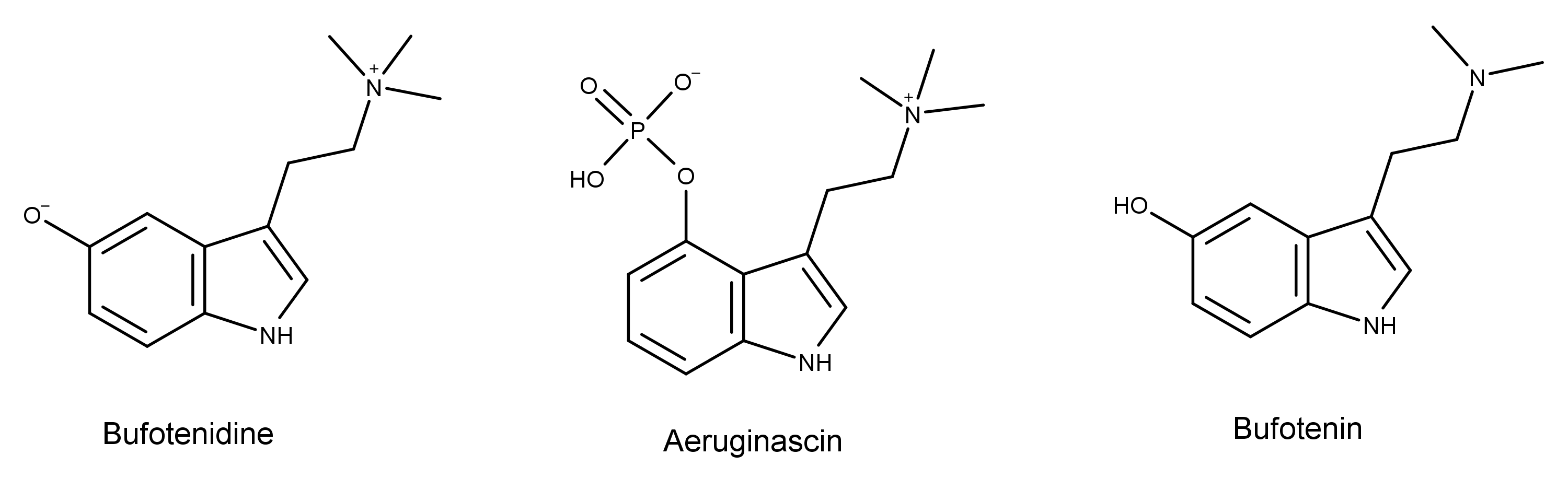
Bufotenidine has a chemical structure similar to the psychedelic mushroom (aka magic mushroom) compound aeruginascin. Bufotenidine is dephosphorylated aeruginascin with the HO- group at the 5-position instead of the 4-position. Bufotenidine is also a derivative of another compound in toad secretions, bufotenin. They are identical except that bufotenidine has a third methyl group on the terminal nitrogen atom.
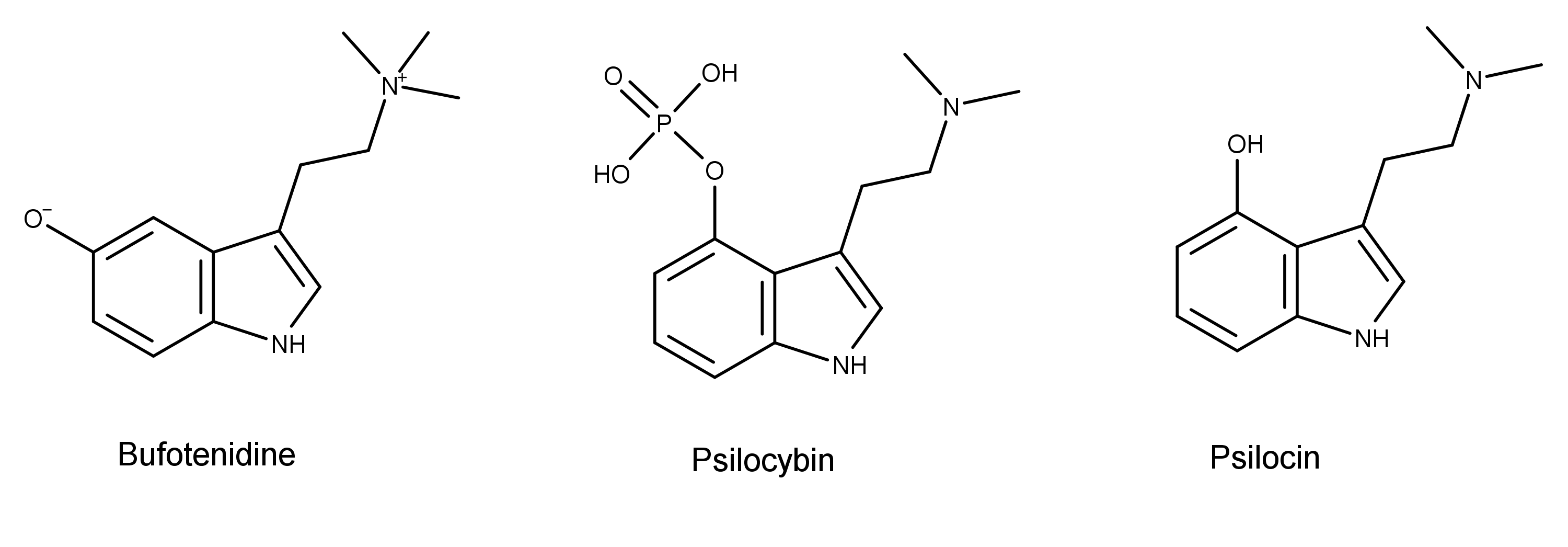
The chemical structures also reveal that bufotenidine is a structural analog the magic mushroom compounds psilocybin and psilocin. In addition to the third methyl group on the terminal nitrogen, bufotenidine is substituted at the fifth carbon. In contrast, psilocybin and psilocin are substituted at the fourth carbon. These seemingly small differences in chemical structure translate into significant differences in their function in the human body.
The Pharmacology of Bufotenidine
Bufotenidine is a selective agonist of the serotonin 5-HT3 receptor.5 As such, bufotenidine is used in scientific research to study the function of 5-HT3. Bufotenidine has not been as well studied compared to its structurally similar counterpart bufotenin.
Traditional Chinese medicine (aka Chan Su) is using toad secretions that contain bufotenidine to treat pain in cancer patients.6,7 Research has shown that the addition of a third methyl group to the ammonium ion (compared to bufotenin) prevents bufotenidine from crossing the blood-brain barrier.6 This explains the ability of bufotenidine to alleviate pain via the peripheral nervous system. However, this barrier also prevents its use in 5-HT3 receptor studies in the brain and other areas of the central nervous system.
A 2017 radioligand binding study found that bufotenidine from the parotid gland of the toad Bufo bufo had a higher affinity for neuronal α7 nicotinic acetylcholine receptors compared with muscular cholinergic receptors.8 This is an interesting finding because the α7 receptor is believed to be involved in long term memory function in the brain.
A study from the early 1970s indicated that bufotenidine isolated from the giant reed Arundo donax caused paralysis in rats.9
The Applications and Potential of Bufotenidine
Overall, when it comes to toad skin secretions, 5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine) is receiving the majority of research attention right now. Investigating the chemistry and pharmacology of bufotenidine presents intriguing possibilities for the curious researcher.