
Norbaeocystin is a psilocybin derivative, i.e., an analog of the psychedelic prodrug psilocybin. It is one of many that comprise a cocktail of compounds in magic mushrooms (aka psychedelic mushrooms).
Understanding the Chemistry and Synthesis of Norbaeocystin
Norbaeocystin has a primary amino group on the ethylamine group that is common to the psilocybin derivatives. However, unlike baeocystin, aeruginascin, or psilocybin, it has no methylation on the ethylamine group. By contrast, baeocystin has a single methyl group at this position, psilocybin has two methyl groups, and aeruginascin has three methyl groups.
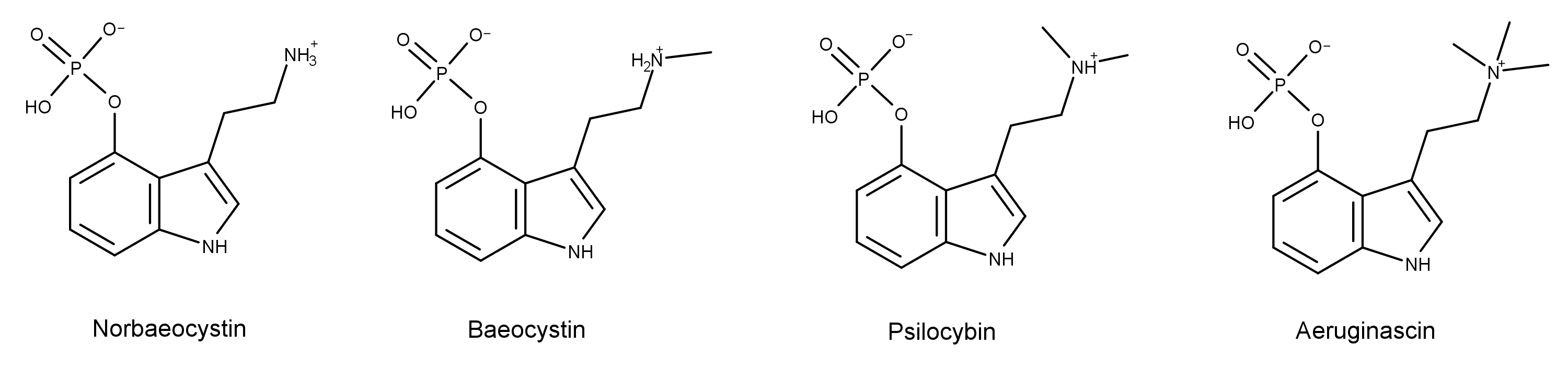 Norbaeocystin was first isolated from submerged cultures of Psilocybe baeocystis.1 In the study, solvent extractions provided material enriched in psilocybin derivatives. Those chemicals were further separated by chromatography to provide pure fractions of norbaeocystin, baeocystin, and psilocybin.
Norbaeocystin was first isolated from submerged cultures of Psilocybe baeocystis.1 In the study, solvent extractions provided material enriched in psilocybin derivatives. Those chemicals were further separated by chromatography to provide pure fractions of norbaeocystin, baeocystin, and psilocybin.
Recently, it was demonstrated that norbaeocystin could be an intermediate in the biosynthesis of psilocybin.2 In vitro tests showed that L-tryptophan is converted to tryptamine by the enzyme PsiD which is then converted to 4-hydroxytryptamine by the enzyme PsiH. PsiD can also synthesize 4-hydroxytryptamine directly from the substrate 4-hydroxy-L-tryptophan. Norbaeocystin is then synthesized by the enzyme PsiK.
The researchers propose that additional methyl groups are added by the enzyme PsiM, generating baeocystin (one methylation) and psilocybin (two methylations). This research establishes the foundation for in vitro enzymatic synthesis of psilocybin and psilocybin derivatives. Alternatively, the desired compounds could be produced by biotechnological production through the genetic engineering of suitable fermentation hosts.
Never Studied and Poorly Understood
For the most part, norbaeocystin has been ignored by the scientific community and it is seldom discussed. Further, its biological effects are not well documented.
Researchers have hypothesized that norbaeocystin is generally found at lower concentrations than psilocybin.3–5 Documented chemotyping of psychoactive mushrooms supports this hypothesis, showing considerably higher amounts of psilocybin vis-a-vis other (unspecified) psilocybin derivatives.
Norbaeocystin is generally regarded as non-psychedelic. However, there is no evidence to support this belief. Furthermore, the absence of psychedelic properties does not exclude it’s potential for therapeutic uses, for example, in modulating the properties of drugs, endogenous neurotransmitters, or both (e.g., serotonin) without an intoxicating effect.
Drawing an analogy to the accepted metabolic pathway in mushrooms for the synthesis of psilocybin,2 one could infer that norbaeocystin is dephosphorylated in the body to form 4-hydroxytryptamine, which is further metabolized. Notably, the proposed 4-hydroxytryptamine compound would differ from serotonin (5-hydroxytryptamine) only by the position of the hydroxy- group. Norbaeocystin is hydroxylated at the 4th position in the molecule, whereas serotonin is hydroxylated at the 5th position.
Different Molecules = Different Drugs
Anecdotal experience reports on sites like Erowid and Bluelight suggest that different species of magic mushrooms result in different effects for the user. However, the majority of discussions on the effects of magic mushrooms center around on just one molecule: psilocybin.
From a chemistry standpoint, it makes sense that structurally different molecules almost certainly provide different effects when ingested by the user. They are different drugs.
Understanding how each psilocybin derivative contributes to the overall effect of a magic mushroom experience would allow researchers to create targeted formulations. Such formulations could be optimized to provide specific properties without undesired side-effects. For example, Wood Lover Paralysis arising from certain species of psilocybin-containing mushrooms.


Very interesting and— timely article.
I am assisting a creative breeder (grower) of mushrooms.Psilocybe cubensis . I am needing to provide him low cost analysis. Can you recommend an article for prep and TLC, Thanks so much. Neale Povey, PhD. npovey@gmail.com