
In the June 4, 2018 issue of The Atlantic author and psychedelics advocate Michael Pollan published a landmark article titled “What It’s Like to Trip on the Most Potent Magic Mushroom.” In the article, Pollan recalls a conversation he had with mushroom expert and mycology legend Paul Stamets after they returned from a mushroom picking trip to gather Psilocybe azurescens. Pollan was looking forward to trying ‘azzies,’ as P. azurescens is also known. However, Stamets dampened his enthusiasm with the comment, “Azzies have one potential side effect that some people find troubling. Temporary paralysis.”
Since mushrooms causing paralysis are often found growing on wood (e.g., P. azurescens and P. cyanescens), the condition has come to be known as wood lover paralysis). Wood Lover Paralysis is characterized by the delayed onset of temporary muscle weakness, paralysis, or both.
Over the years, anecdotal experience reports such as those on Shroomery.org (examples here and here) have confirmed the temporary loss of muscle control after ingesting these and other species. Also, it is important to note the similarities between Wood Lover Paralysis and the autoimmune neuromuscular disease myasthenia gravis (MG). The symptoms of MG include weakness in the skeletal muscles which are responsible for moving the body (such as the arms and legs) and breathing. The latter is particularly important because a misdiagnosis of Wood Lover Paralysis as MG in an emergency room situation could lead to unnecessary intubation of the patient.
Science does not understand how or why Wood Lover Paralysis occurs, and there is currently no ongoing research to try to unravel the mystery surrounding this condition.
The Science Behind the Aeruginascin Hypothesis
Others have suggested a chemical cause for Wood Lover Paralysis: namely, that a compound other than psilocybin or psilocin is the cause. Magic mushrooms contain several compounds including psilocybin, psilocin, norpsilocin, baeocystin, norbaeocystin, and aeruginascin. When it comes to understanding Wood Lover Paralysis, aeruginascin’s chemical structure presents an intriguing and feasible piece that fits the puzzle. Despite the mystery of Wood Lover Paralysis and an absence of research into its causes and mechanisms, it is possible to form a compelling hypothesis by studying the chemical structure of aeruginascin and comparing it to similar compounds with known effects.
Evaluating the Dose Hypothesis
If the dosage or potency of psychoactive chemicals a person receives from eating magic mushrooms is causing Wood Lover Paralysis, then it follows that people eating species that grow on non-wood substrates would experience it, too. However, this is not the case. For example, experience reports show that people who consume large amounts of P. cubensis (which contain lots of psilocybin and don’t grow on wood) do not experience paralysis.
Structural Similarity Between Aeruginascin and Psilocybin
Aeruginascin is a compound present in several species of magic mushrooms.1 The chemical structure of aeruginascin is similar to psilocybin, the primary psychedelic prodrug in magic mushrooms. Aeruginascin has three methyl groups on the ethanolamine moiety while psilocybin has two (Figure 1). The additional methyl group creates a positively charged trimethylammonium group on aeruginascin compared to a dimethylamine group on psilocybin. Both of these compounds are structural analogs of the neurotransmitter serotonin (Figure 2).
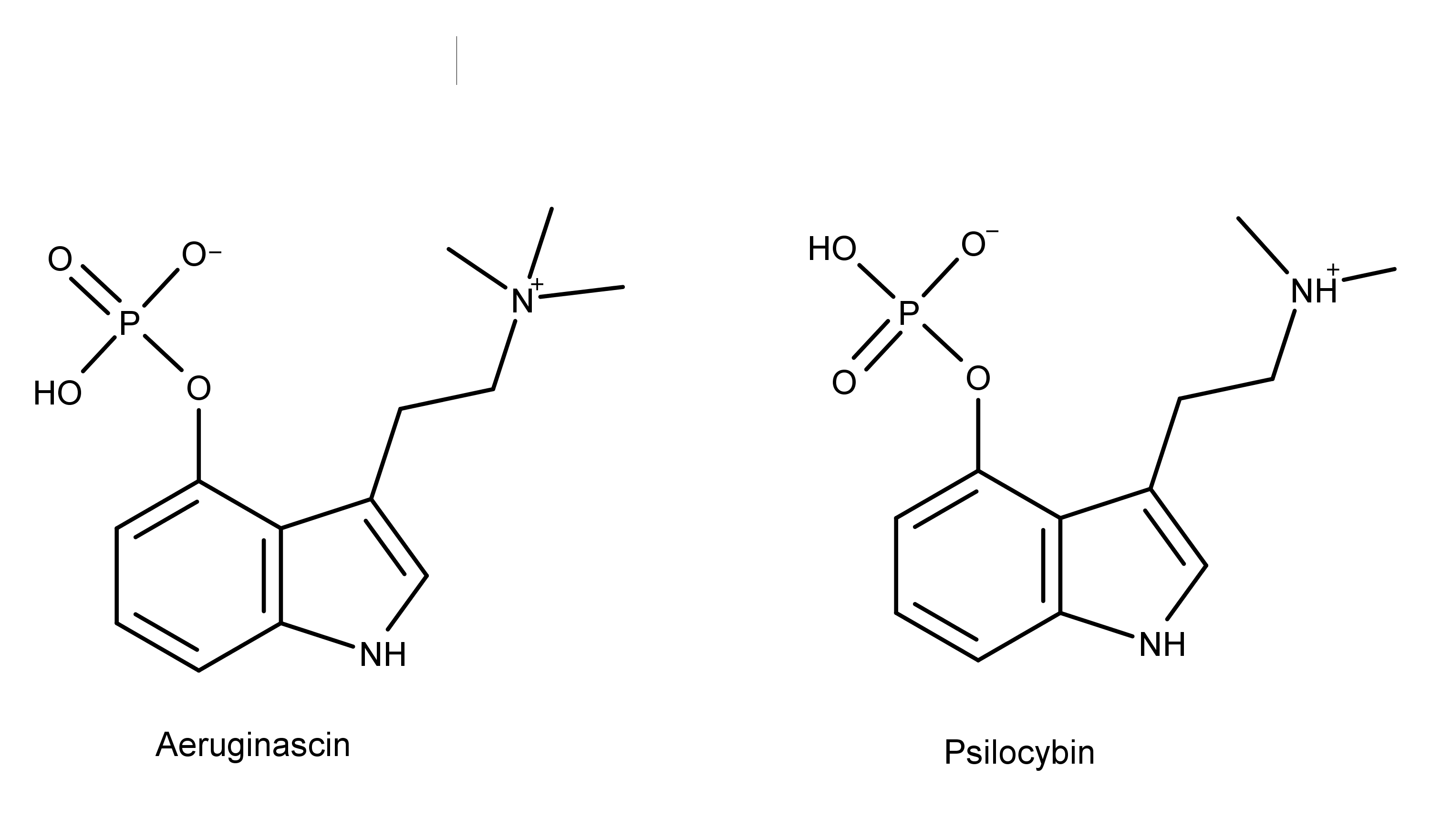
Figure 1: The chemical structures of aeruginascin and psilocybin. Notice that aeruginascin has three methyl groups and psilocybin has two.
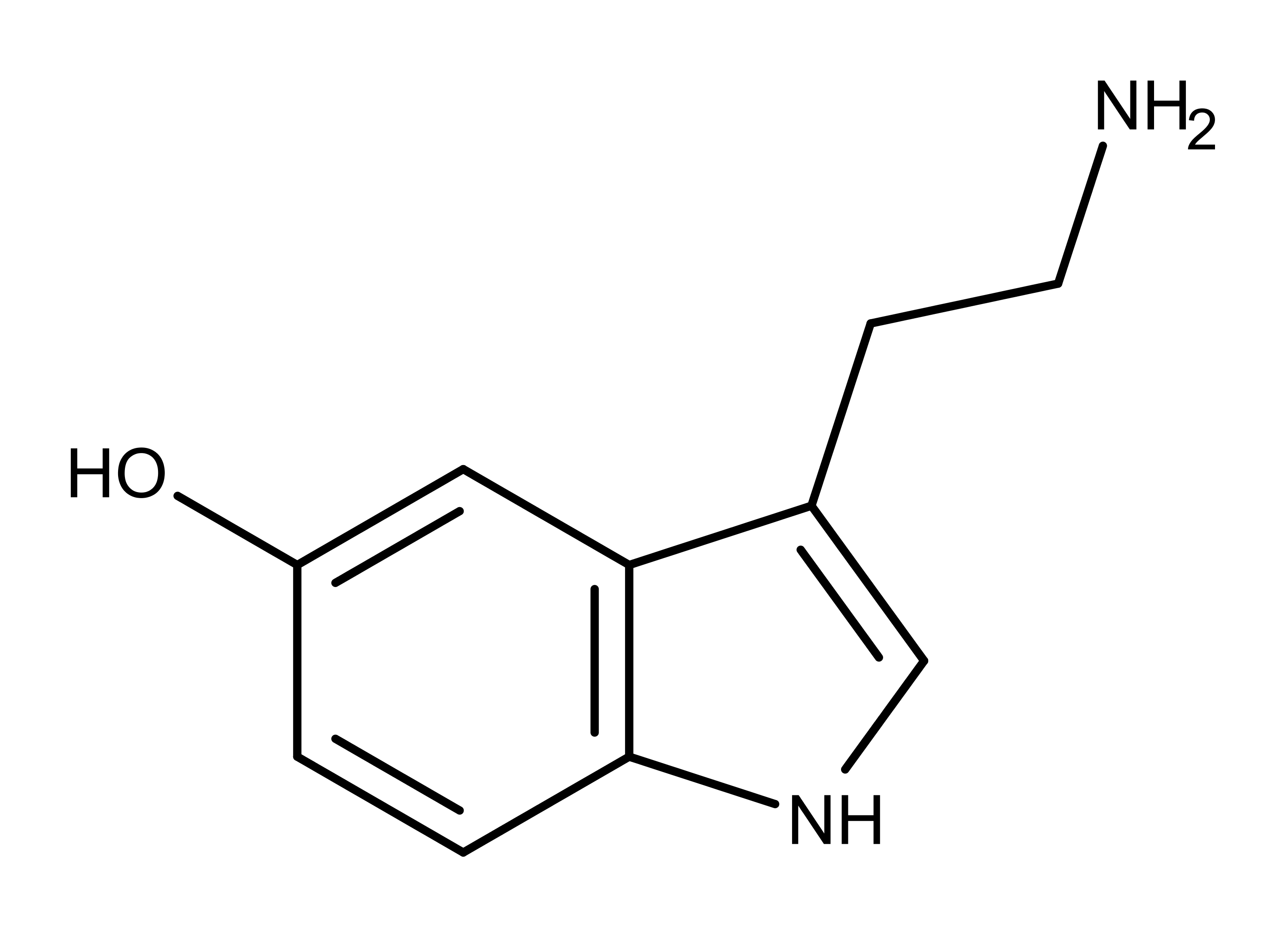
Figure 2: The neurotransmitter serotonin, a structural analog of aeruginascin, psilocybin, bufotenin, and bufotenidine.
The structural similarities between aeruginascin and psilocybin could indicate that they have similar metabolic pathways, bind to the same receptors, and elicit similar effects.
Structural Similarity to Bufotenin and Bufotenidine
The same structural difference exists between the toad venoms bufotenin and bufotenidine (also known as 5-HTQ) (Figure 3) which are also structural analogs of aeruginascin, psilocybin, and serotonin. Notice that bufotenin and bufotenidine differ by only a single methyl group, just like aeruginascin differs from psilocybin. Bufotenin is a psychoactive compound. However, bufotenidine with its one additional methyl group is known to cause paralysis.2 The difference in these effects based on the presence or absence of a third methyl group can be extrapolated to explain how aeruginascin could cause Wood Lover Paralysis.
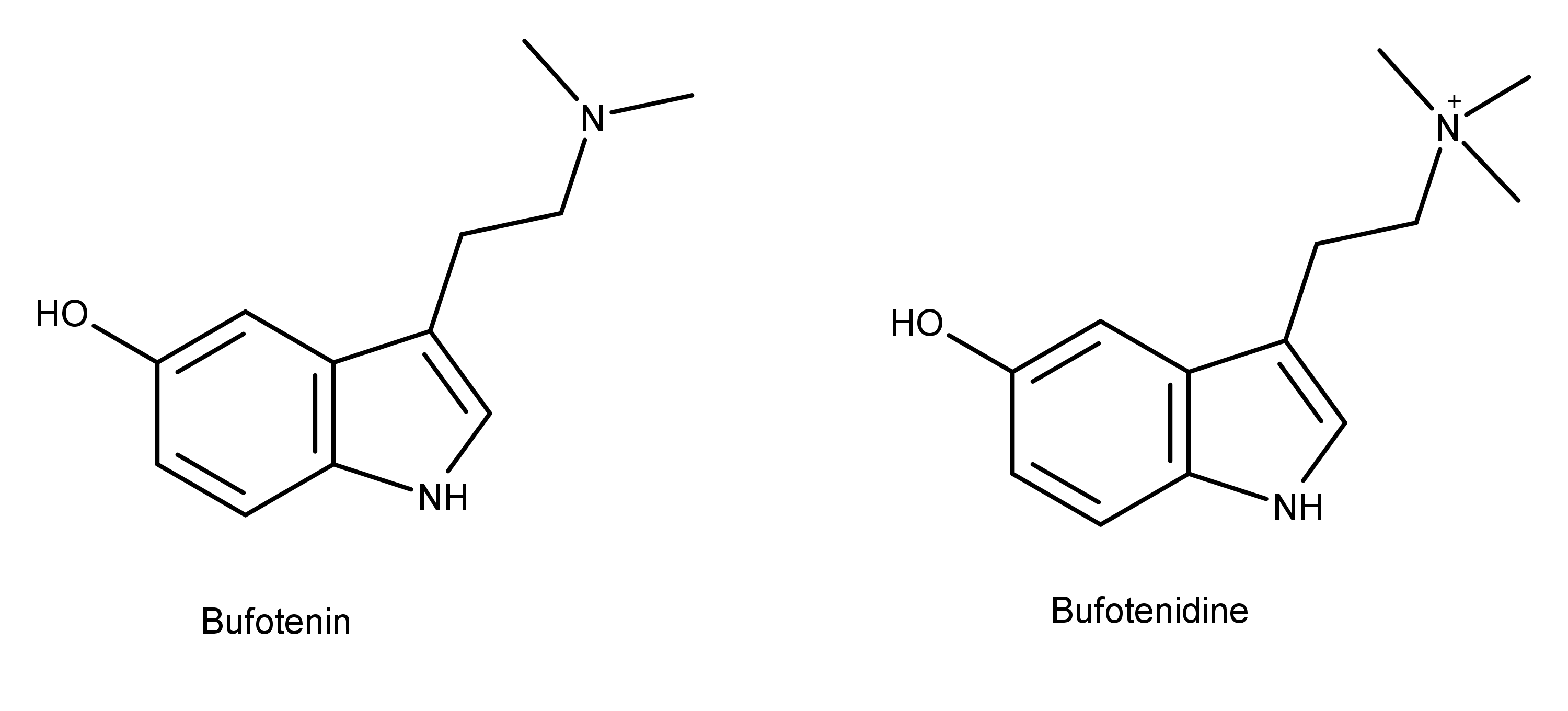
Figure 3: The chemical structures of bufotenin and bufotenidine. These compounds are close structural analogs of psilocybin and aeruginascin (Figure 1).
At his point, the aeruginascin hypothesis requires a discussion about metabolically active and inactive forms of molecules. It is common in nature that some phosphorylated molecules are prodrugs of the metabolically active versions. For example, the body removes the phosphate group from the prodrug psilocybin, transforming it into psilocin which is the psychoactive compound (Figure 4). Therefore, it stands to reason that aeruginascin could be a prodrug that the body dephosphorylates to the biologically active compound. Science has yet to discover and name a dephosphorylated version of aeruginascin. In contrast, phosphorylated versions of bufotenin and bufotenidine are not known, either. So, there is much mystery remaining just in terms of isolating the basic compounds.
The toad venoms discussed herein also differ from aeruginascin and psilocybin by the location and type of their substituent groups. Bufotenin and bufotenidine have a hydroxyl group on the 5th carbon while aeruginascin and psilocybin have a phosphate group on the 4th carbon (see here for how to number the carbons). Assuming aeruginascin is dephosphorylated in the body into the active form like the conversion on psilocybin to psilocin, then all the substituents in question become hydroxyl groups. In this scenario, aeruginascin only differs from bufotenin and bufotenidine by where the hydroxyl group is on the ring. This difference could be just as crucial to the physiological behavior of these molecules as the number of methyl groups discussed earlier. Further research will answer this question.
Other Considerations Suggesting Aeruginascin is Involved in Wood Lover Paralysis
The chemistry of aeruginascin also determines where it can travel in the human body after ingestion. Due to its large trimethylammonium group, it is unlikely that aeruginascin can penetrate the blood-brain barrier.3 Because of this segregation, the effects of aeruginascin are confined to the peripheral nervous system (PNS) outside of the brain and spinal cord, making it a candidate for the cause of Wood Lover Paralysis.
Scientists have the understanding that the psychedelic effects of compounds like psilocin and LSD are mediated by the serotonin receptor subtype 5-HT2A.4 The 5-HT2A receptor is concentrated in regions of the forebrain, primarily the cortical areas, caudate nucleus, nucleus accumbens, olfactory tubercle, and the hippocampus.5 A different serotonin receptor subtype is the 5-HT3 receptor. It is located throughout the body including the brain but also widely distributed throughout the PNS.6
Of the 7 known serotonin receptor families, 5-HT3 is unique in that it works via a different mechanism than the others. Serotonin receptor families 1, 2, and 4-7 are G-protein coupled receptors that have a distinct structure of 7 coils that traverse the cell membrane.7 When these receptors bind serotonin and other compounds, they activate signaling pathways in the cell that use phosphatidylinositol and cyclic AMP (cAMP). The 5-HT3 receptor, however, works via a ligand-gated sodium and potassium channel. For these receptors, the binding of serotonin opens ion channels, allowing sodium and potassium to enter the cell, leading to an excitatory response. The ion-gated mechanism by which 5-HT3 receptors work is more like the way acetylcholine binds to neurons at neural-muscle junctions in the body, creating signaling for muscles to contract.
Studies have found that the frog venom bufotenidine (the one that causes paralysis) binds to the 5-HT3 receptor with ten times the affinity of serotonin.8 Recall the structural similarities between bufotenidine and aeruginascin (Figure 5). If aeruginascin is dephosphorylated in the body and carbon 4 receives a hydroxyl group as a result, then the only difference between the paralysis-causing bufotenidine and the Wood Lover Paralysis-causing candidate aeruginascin is whether carbon 4 or 5 has the hydroxyl group. Both molecules have the charged and reactive trimethylammonium group, so it is feasible they may affect the same receptor and cause the same effect. Also, based on its chemical structure, bufotenidine may be confined to the PNS and not be able to cross the blood-brain barrier, just like aeruginascin.8
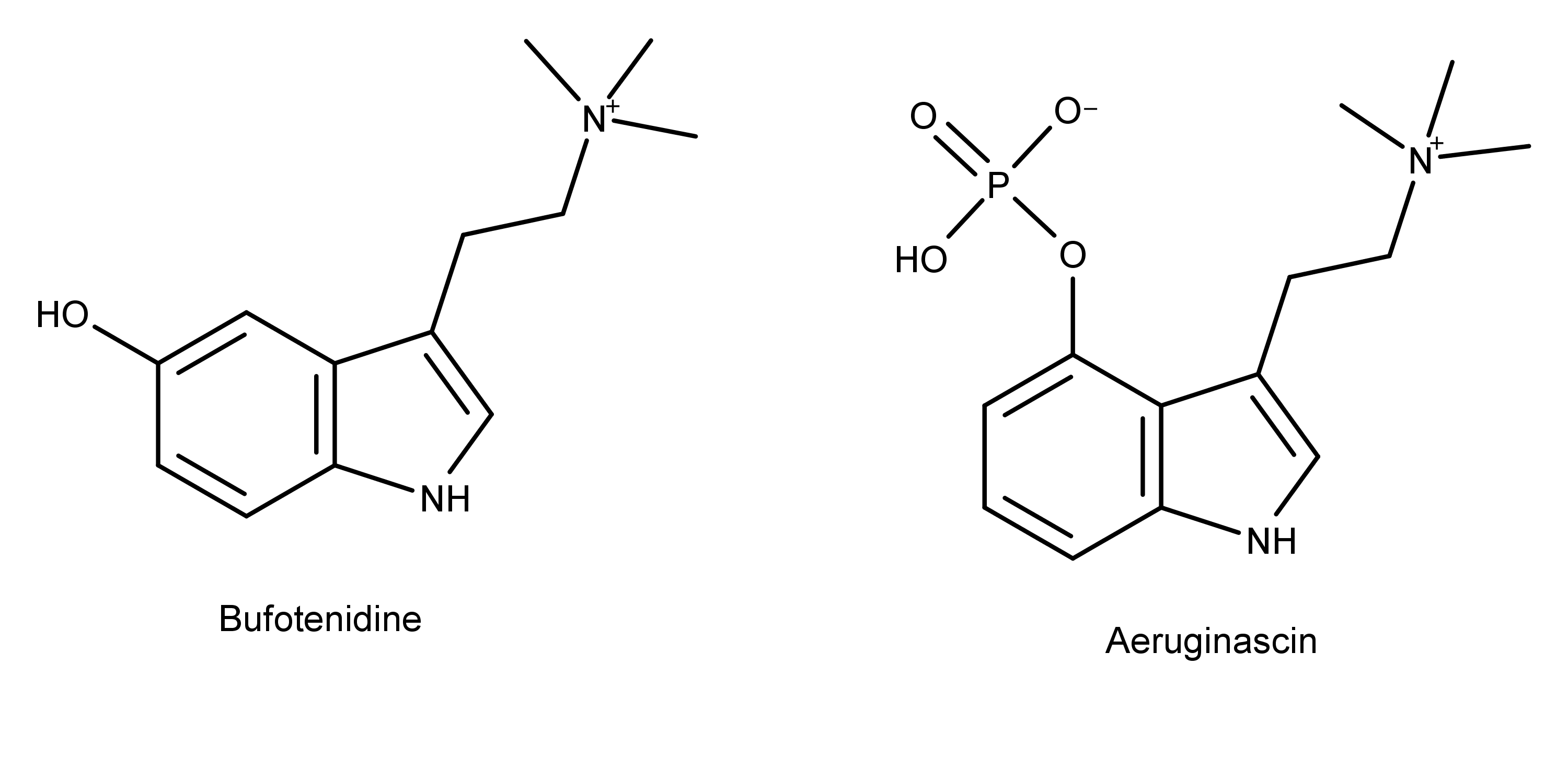
Figure 5: The chemical structures of the toad venom bufotenidine and the psilocybin derivative aeruginascin.
Conclusion
Nature gives hints to a possible cause of Wood Lover Paralysis in the chemical structure of aeruginascin. Comparing aeruginascin to structurally similar compounds with known effects provides more clues. Structural similarities between aeruginascin and the known paralytic toad venom bufotenidine are one factor that may help explain the cause of Wood Lover Paralysis.
Also, scientists know that the neurotransmitter serotonin and its structural analogs such as bufotenin and psilocin cross the blood-brain barrier and bind to the abundant 5-HT2A receptors located mostly in the forebrain. Binding to this receptor causes the effects associated with psychedelics. However, due to its chemical structure, bufotenidine is probably confined to the peripheral circulation in the body where the tissues contain 5-HT3 receptors, explaining its paralytic effects. A dephosphorylated form of aeruginascin could conceivably work in the same way to cause Wood Lover Paralysis.

Am I the only one who sees the gaping hole in the central argument of this article?!
What evidence is there that ps Azurescens or ps Cyanescens contains aeruginascin? In addition Gartz’s study of accidental ingestion of the mushroom that Is known to contain aeruginascin Includes no mention of paralysis.
Editor?
Based on the other article I read it seems they are looking for it in these mushrooms though its possible it could be some other unknown derivative similar to this. But I know more than one person that feels lethargic and weak during a mushroom experience and a high dose of these might amplify that effect considerably given how strong they are, although cyans being relatively common around Portland and high doses being easy to obtain, even during a white-out type situation no-one I know has experienced muscle paralysis.
I know many people that have experienced this, myself at least 10-times. I live in Portland. In my experience, it is more common with azurescens than cyans.
Gartz also claims wood lover paralysis is “a myth”. He doesn’t believe in it. His credibility is definitely in question as an expert on wood lover paralysis. There definitely have been reports of paralysis from Inocybe aeruginascens. Aeruginascin has in fact been found in cubensis. It is prudent to assume it may be present in cyans and azzies as well.
I can’t avoid to compare the structure of the quarternary amine aeruginascin, to other quarternary amines, like salts of tetramethyl-ammonium, tetramethyl-ammonium, several related phase-transfer catalysts, related cathionic detergents and herbicides, the plant-growth regulator chlormequat chloride, choline and peripheral neuromuscular blocking agents, like pancuronium and succinyldicholine etc. The action of these substances, are generally connected to their effects on muscarinic or nicotinic receptors, peripherally simulating or blocking the effect of acetylcholine. Paralysis, dominantly can be related to longer depolarisation or blocking of the nicotinic receptors of the skeletal muscle cells, and inducing contraction and fasciculation, by the release of calcium ion,… Read more »
I have also been considering this. IMO it is either the 5HT3 receptor/bufotenidine theory or the muscarinic acetylcholine receptors. The drug Diphenhydramine has been used as a treatment on the streets. It is a sodium channel blocker, a potent antimuscarinic and anti histamine. We have ruled out the histamine H1 receptor and are left with the ligand-gated sodium and potassium channel and the muscarinic acetylcholine receptors. There is another thread on this topic. Look up “wood lover paralysis –the antidote” Come join the conversation!
Seems to me there’s a big, huge problem with this hypothesis — Aeruginascin is only known in Inocybe aeruginascens and Pholiotina cyanopus.
If Psilocybe azurescens does not contain aeruginascin, then aeruginascin can’t be the cause (ot at least the sole cause) of WLP.
My wife consumed 2 grams of cultivated Psilocybe cubensis and experienced WLP on the left side of her body. We were sure that it was a stroke, but CAT scan determined that it wasn’t. She slowly started recovering from the paralysis in the hospital. She described her arm and leg as like lead. Notable to this was the fact that the mushrooms were grown in a controlled environment with no noticeable contamination.