
Baeocystin is a chemical compound found in psychedelic mushrooms (aka magic mushrooms). From a chemical standpoint, baeocystin is closely related to psilocybin. The two molecules differ by one methyl group. Baeocystin is [3-(2-methylaminoethyl)-1H-indol-4-yl] dihydrogen phosphate. Psilocybin is 3-(2-dimethylaminoethyl)-1H-indol-4-yl] dihydrogen phosphate. Figure 1 illustrates this structural difference.
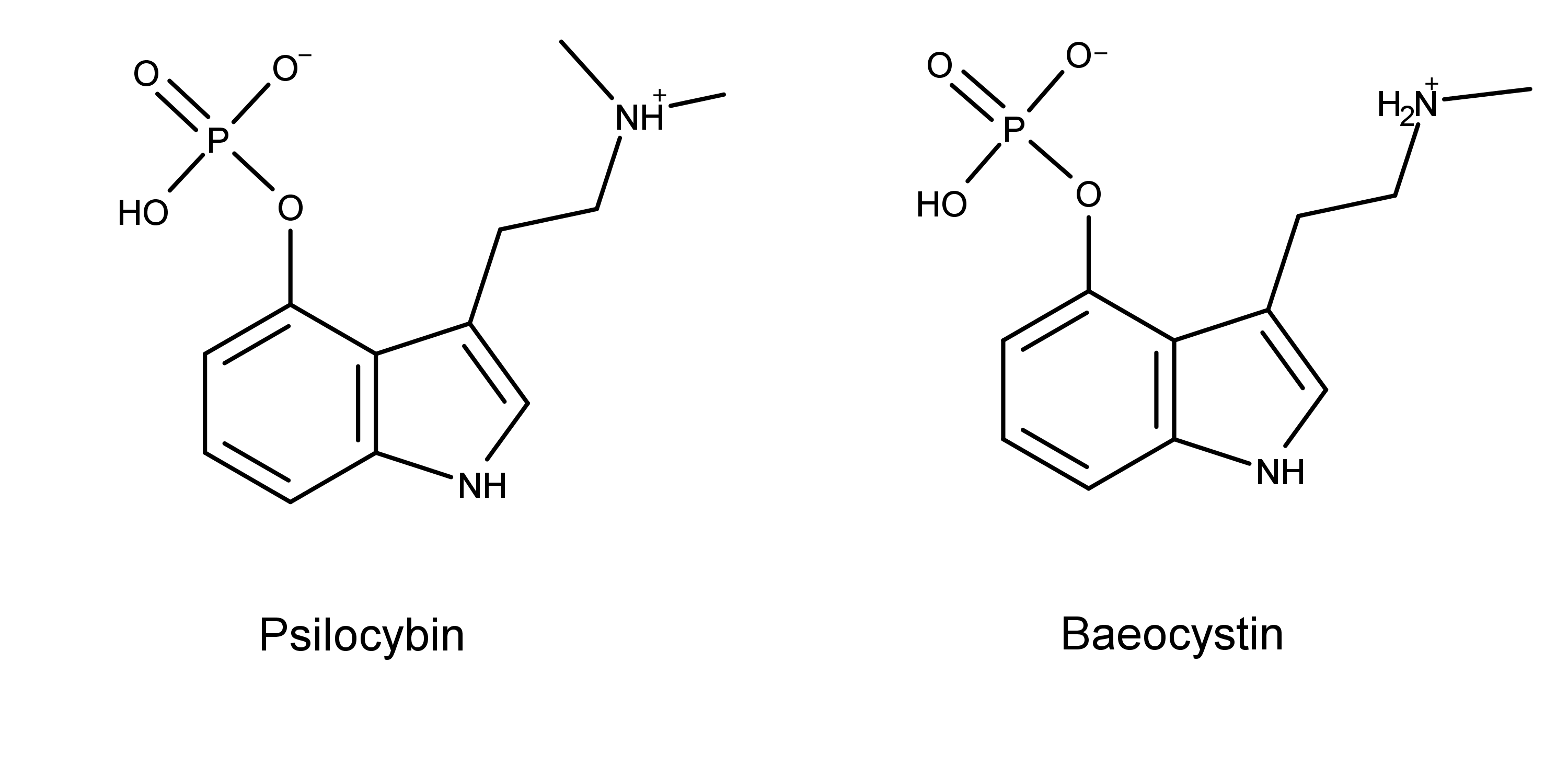
Figure 1: The chemical structures of psilocybin and baeocystin. Note the two methyl groups on the ethyl amino group in psilocybin compared to one on baeocystin.
Because of the similarities in their chemical skeletons, baeocystin can be categorized as a psilocybin derivative or analog. Baeocystin is often described as either the N-demethylated derivative of psilocybin or the phosphorylated derivative of 4-HO-NMT (4-hydroxy-N-methyltryptamine).
Baeocystin was first isolated by Leung and Paul in 1968 from the mushroom Psilocybe baeocystis (hence the name).1 Later, other researchers isolated it from Psilocybe semilanceata, Panaeolus renenosus, Panaeolus subbalteatus, and Copelandia chlorocystis.2,3 Baeocystin was first prepared synthetically in the lab by Franz Troxler, Sailor, and Albert Hofmann in 1959.4
What We Know About the Effects of Baeocystin and Its Pharmacology
The scientific community has very little data on baeocystin or its human pharmacology. At best, there are anecdotal reports by people taking the drug. For example, in the book Magic Mushrooms Around the World, author Jochen Gartz refers to a report that “10 mg of baeocystin were found to be about as psychoactive as a similar amount of psilocybin.” 5 Gartz also reported that his experience taking 4 mg of the pure drug caused “a gentle hallucinogenic experience.”
Aside from these accounts, no one has quantified the effects of baeocystin – alone or in combination with other active magic mushroom species. Although the presence or absence of a methyl group seems like a small structural change, these sorts of chemical differences often result in significant changes in pharmacology.
Another example is the comparison between amphetamine and methamphetamine. These compounds differ only by one methyl group on their respective ethyl amino groups. That difference results in dramatically different activity between the two molecules.6
Future Research
Future research in this area will benefit from shifting the focus from magic mushrooms to the active compounds within them. The current state of the art for psilocybin technology can be improved by isolating each of the individual molecules and studying how they affect cellular receptors (e.g., serotonin) alone and in combination with other compounds.
