The Chemistry of 2C-H
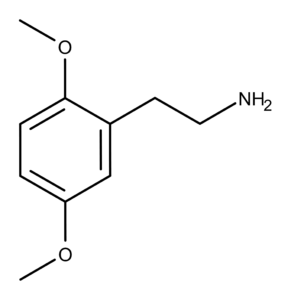
The compound 2C-H is a phenethylamine that was first synthesized by Johannes Buck of the Burroughs and Wellcome Company in 1932.1 In his paper, Buck reported that the colorless, viscous liquid rapidly absorbs carbon dioxide in the air, changing into a carbonate solid. He also described it as “rather slightly soluble in water,” resulting in a strongly alkaline solution.
Buck added, “They [the beta-phenlethylamines] are miscible with alcohol, benzene and ether. The odors are much fainter than would be expected. The mediocre yields in some cases are doubtless due to nuclear halogenation, a not infrequent accompaniment to the [Albert] Hofmann reaction.”
2C-H is compound #32 in Alexander and Ann Shulgin‘s book PiHKAL.2 They describe their synthesis of the compound, starting with 2,5-dimethoxybenzaldehyde, nitromethane, and anhydrous ammonium acetate. Like Buck, the Shulgin’s reported that the free base form of 2C-H “picked up carbon dioxide rapidly when exposed to the air.”
The Pharmacology of 2C-H
There have been no clinical trials of 2C-H using humans. At the time, the Shulgin’s suspected that most of the orally-ingested 2C-H would fall victim to monoamine oxidase (MAO) enzymes during the first-pass metabolism.2 The Shulgin’s entry for this compound in PiHKAL shows the dosage and duration of effects as unknown.
However, a 2019 study by Wagmann et al. found that among twenty-two phenethylamine-derived psychoactive compounds, 2C-H was the most potent inhibitor of MAO-B with an IC50 value of 1.7 µm.3 This value equates to a 50% reduction in the enzyme activity. In contrast, 2C-H did not show any statistically significant ability to inhibit MAO-A.
A 2015 study by Rickli et al. investigated the binding affinities of several 2C drugs, their NBOMe analogs, and LSD at monoamine receptors4 (see the data table at the end of this article). They reported that all the compounds exhibited a high binding affinity for 5-HT2A and 5-HT2C receptors (Ki < 1µm) except for 2C-H and mescaline. Also, all the compounds potently activated 5-HT2A, typically more than LSD (EC50 < 1µm), except for 2C-H and mescaline. All the compounds were potent activators of 5-HT2B, with the exception of 2C-H, mescaline, mescaline-NBOMe, and LSD. And for 5-HT1A, all the 2C compounds bound potently (Ki < 0.52 µM) except for 2C-N and mescaline.
In August 2020, a paper by Flanagan et al. reported that 2C-H is the pharmacophore of anti-inflammatory activity in rats.5 This discovery reveals that 2C-H has the chemical characteristics essential for the anti-inflammatory response via 5-HT2A. Overall, their data indicated that activating 5-HT2A is “necessary and sufficient for the anti-asthma effects of psychedelics” in rats.
The data also showed that a psychedelic compound’s ability to prevent asthmatic symptoms does not involve activating calcium mobilization in cells downstream from 5-HT2A. By integrating their results with behavioral studies in the literature, the authors hypothesized that a particular psychedelic’s anti-inflammatory effects would be at a lower dose than what is needed to produce behavioral effects.
The Applications and Potential of 2C-H
The Shulgin’s explain in PiHKAL that 2C-H is “…one of the most magnificent launching pads for a number of rather unusual and, in a couple of cases, extraordinary drugs. In the lingo of the chemist, it is amenable to ‘electrophilic attack at the 4-position.’ And, in the lingo of the psychopharmacologist, the ‘4-position is where the action is.’ From this (presumably) inactive thing have evolved end products such as 2C-B, 2C-I, 2C-C, and 2C-N. And in the future, many possible things as might come from a carbinol group, an amine function, or anything that can stem from a lithium atom.”
Clearly, abundant research opportunities exist for studying 2C-H.
Receptor Binding Affinity Data
| Receptor | Ki (nM) | Species | Note | Ref. |
|---|---|---|---|---|
| 5-HT1A | 70 | Human | 4 | |
| 5-HT2A | 1600 | Human | 4 | |
| 5-HT2C | 4100 | Human | 4 |