In 1963, chemists Albert Hofmann and Franz Troxler patented their discovery of 4-AcO-DMT (psilacetin) along with other indole esters they had synthesized.1 Just as Hofmann put LSD aside for five years after synthesizing it in 1938, unaware of its psychedelic properties, psilacetin was patented but shelved. According to the United Nations Office on Drugs and Crime, synthetic tryptamines like psilacetin began appearing in illicit drug markets throughout the 1990s.2
The Chemistry of Psilacetin (4-AcO-DMT)
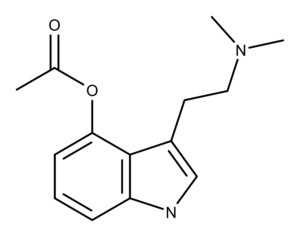
Psilacetin is a structural analog of the psychedelic mushroom (aka psilocybin mushroom or magic mushroom) compound psilocybin. Psilocybin is a prodrug of psilocin, and psilocin is an analog of the neurotransmitter of serotonin. Chemically, psilacetin is O-acetylated psilocin, whereas psilocybin is O-phosphorylated.
Following 20 years of inactivity, the chemical community began publishing new data about psilacetin in the late 2010s. Building on the work Nichols and Frescas did in 1999 scientists solved the crystal structure of 4-AcO-DMT fumarate in March 2019.3 Chadeayne, et al. demonstrated that the solid-state structure is an asymmetric unit containing one 4-acetoxy-N,N-dimethyltryptammonium cation, and one 3-carboxyacrylate anion. Despite its use as a research chemical in the illicit drug market, this work was the first conclusive structural characterization of the molecule.
In a follow-up to this study, the same research team defined the crystal structure of a new solvate form of 4-AcO-DMT fumarate, bis(4-acetoxy-N,N-dimethyltryptammonium) fumarate.4 The crystal structure consists of two protonated psilacetin molecules that are charge-balanced by one fumarate dianion. This new solvate form of psilacetin is an important discovery because it opens the door to more options for drug development. In 2021, the team built further on this work when they published crystal structures of the fumarate salts of the methyl-ethyl-, methyl-allyl, and diallyl variants of psilacetin.5
The Pharmacology of 4-AcO-DMT
In their 1963 patent, Hofmann and Troxler described indole acetate esters like 4-AcO-DMT as serotonin receptor antagonists. However, over the years, scientists have come to classify many of them as prodrugs that are inactive on their own by way of the analogy of psilocybin being a prodrug of psilocin. The renowned psychedelic researcher Dr. David Nichols has suggested that psilacetin, like psilocybin, is a prodrug of psilocin. In their 1999 work, Dr. Nichols and Dr. Stewart Frescas synthesized the fumarate salt of psilacetin.6
A 2017 substance abuse study using rodents suggested that a single administration of 4-AcO-DMT prevents and reverses heroin and nicotine addictions.7 The authors of the study theorize the mechanism involves preventing the up-regulation of brain-derived neurotrophic factor via serotonin 5-HT2A receptor signaling.
A 2022 study by Glatfelter et al. examined the receptor affinities of psilocybin analogs with different N-alkyl substitutions or ring-substitutions in mice.8 Results showed that these analogs exhibited low to mid nM affinities for 5-HT (serotonin) receptors and other non-serotonergic targets. In vivo experiments with mice revealed that 4-AcO-DMT may have been converted to psilocin, as evidenced by induced head twitch responses (HTRs). 4-AcO-DMT also demonstrated psychedelic activity on its own, with a binding affinity to 5-HT2A receptors. The study concluded that 4-AcO-DMT may serve as an alternative prodrug for psilocin, suggesting its potential as a psychedelic compound. The authors recommended further research comparing the pharmacokinetics and metabolism of psilocybin and 4-AcO-DMT to confirm their conversion to psilocin in vivo. In the same year, another paper by Glatfelter et al. reported that 4-AcO-DMT was less potent than psilocin in mice which would be expected from a prodrug.9 The study also found that 4-AcO-DMT had nanomolar affinity most of the serotonin receptors and induced dose-related increases in HTRs.
In 2022, Zhai et al. used pooled human liver microsomes to identify the metabolites of 4-AcO-DMT for the first time.10 They found 15 metabolites (as well as unmetabolized 4-AcO-DMT) using MS/MS fragmentation patterns. The most abundant metabolite was psilocin (via hydrolysis), confirming that 4-AcO-DMT is a prodrug of psilocin. The microsomes produced other metabolites via the pathways of hydroxylation, N-demethylation, oxidation, and conjugation with glucoronic acid. The authors noted that the 4-AcO-DMT standard they used was only 90% pure and contained impurities including psilocin, 4-AcO-DMT N-oxide, and psilocin N-oxide.
More recently, a 2024 paper by Jones et al. examined the time-course and plasma concentrations of psilocin by using liquid chromatography–tandem mass spectrometry (LC–MS/MS) after the intraperitoneal (IP) administration of psilacetin fumarate or psilocybin.11 The comparisons of the time courses exposure revealed that at 15 minutes post-injection, psilocybin led to 10–25% higher psilocin concentrations than psilacetin. The half-life of psilocin remained consistent at around 30 minutes, regardless of whether it originated from psilocybin or psilacetin. Overall, psilocin exposure from 4-AcO-DMT fumarate was approximately 70% of that observed with psilocybin. The authors stated that this is the first study looking at in vivo evidence that 4-AcO-DMT is a prodrug of psilocin.
Updated January 23, 2024.