4-HO-DiPT Chemistry
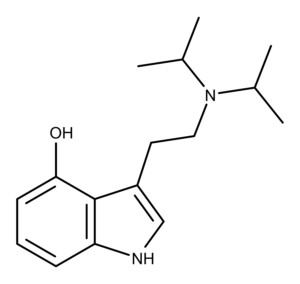
4-hydroxy-N,N-diisopropyltryptamine is a non-naturally occurring structural analog of psilocin in which both N,N-di-methyl groups of psilocin are replaced with isopropyl groups (Figure 1). In 1977, David Repke first synthesized 4-HO-DiPT hydrochloride.1 Shulgin and Shulgin subsequently reported synthesizing 4-HO-DiPT hydrochloride in TIHKAL.2 Neither Repke nor the Shulgins isolated or characterized the freebase form of 4-HO-DiPT or any salts other than the hydrochloride.
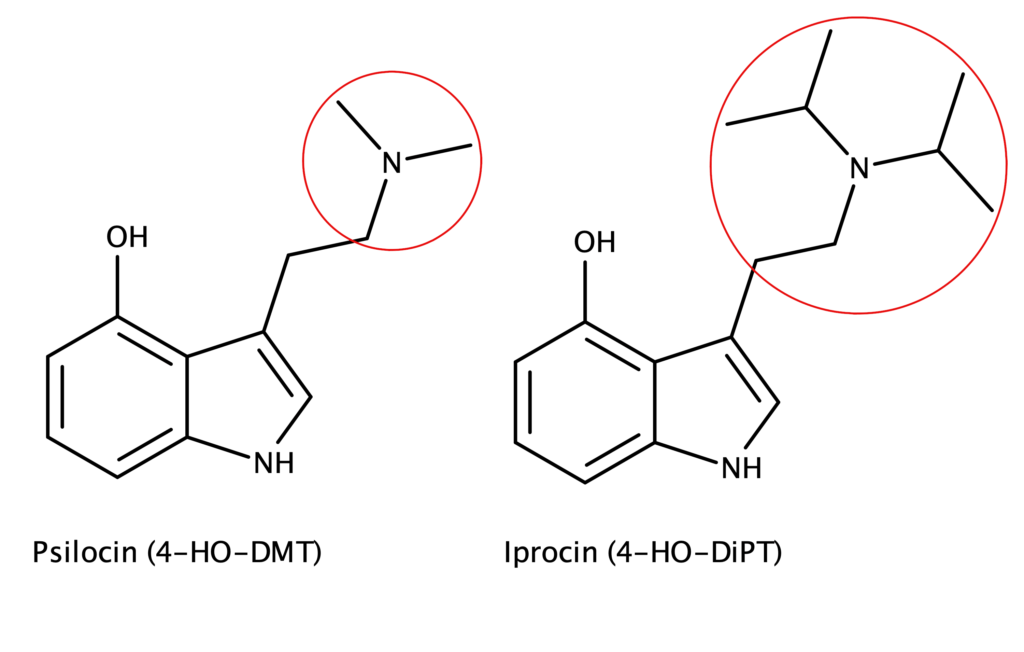
Figure 1: The chemical structures of Psilocin and Iprocin.
On December 30, 2021, 4-glutarato-DiPT, a “hemiester” prodrug of 4-HO-DiPT was reported by Bryson in the patent literature.3 In that publication, the authors distinguished between three different forms of the 4-glutarato-DiPT, including (1) the zwitterionic form, (2) the hydrochloride salt, and (3) a neutral form having both a protonated carboxylic acid and a freebase amine, shown below in Figure 2. In September of 2022, Naeem et al. published the crystal structures of the ethanol and methanol solvates of 4-glutarato-DIPT – both in the zwitterionic form, i.e. “with a protonated tertiary ammonium group and a deprotonated carboxylate within the glutarato group.”4 Notably, the form reported by Naeem et al. was not reported in the Bryson patent publication and Naeem et al. explicitly highlighted the “importance of single-crystal X-ray diffraction studies when characterizing tryptamine salts because they can occur in a variety of forms that are often not appreciated by other means of characterization.”
Following up on the 2021 patent publication,3 Bryson et al. published a paper in 2024 describing the synthesis and biological activity of the hydrochloride salt of 4-glutarato-DiPT, i.e. 5- ((3-(2-(diisopropylamino)ethyl)-1H-indol-4-yl)oxy)-5-oxo-pentanoic acid hydrogen chloride, aka RE104.5 Notably, they reported obtaining the product in greater than 100% yield (based on moles of 4-HO-DiPT starting material) and also noted that the product contained 9.4% pyridine and 9.6% dichloromethane, i.e. 19% teratogenic impurities. To remove the ~20% impurities, the authors subsequently washed their isolated material with additional dichloromethane and ether before drying it under vacuum to afford a second form, which Bryson et al. called “the solid zwitterion (IUPAC name: 5-[(3-{2-[bis(1-methylethyl)azaniumyl]ethyl}-1H- indol-4-yl)oxy]-5-oxopentanoate),” which was further described as follows: “purity 98.3% with 0.48% total impurities; Melting point (differential scanning calorimetry): 132 °C.” Bryson explained that the “solid zwitterion” was characterized by proton and carbon NMR in DMSO-d6 and that those NMR data were found to “conform” to the hypothesized “zwitterion” structure.
4-HO-DiPT Pharmacology
In TiHKAL, Alexander and Ann Shulgin described their experience consuming (orally, 15-20 mg) 4-HO-DiPT hydrochloride:2
I truly doubt that there is another psychedelic drug, anywhere, that can match this one for speed, for intensity, for brevity, and sensitive to dose, at least one that is active orally.
They reported that the effects began within 15 minutes and lasted about 2-3 hours.
A 2016 study by Dinger et al. reported the inhibitory properties (IC50) of 4-HO-DiPT at CYP (Cytochrome P450) isozymes.6 The data revealed that 4-HO-DiPT had inhibitory affinity for all the CYP isozymes tested, with the greatest inhibition occurring at CYP2D6 (IC50 = 9.6 μm; CYP2D6 is one of the most important CYPs, responsible for metabolism of foreign substances in the body). In addition, the authors noted that 4-HO-DiPT inhibited CYP3A with a similar affinity as clarithromycin (IC50 = 115 and 116 μm, respectively), which elicits its antibiotic activity via inhibition of CYP3.7
In 2020, Adam Klein and his research team reported both in vitro calcium mobilization assays and head-twitch response (HTR) data for 4-HO-DiPT hydrochloride.8 Like psilocin, 4-HO-DiPT is a 5-HT2A agonist and it produces the HTR in mice. Notably, 4-HO-DiPT is more selective than psilocin at the serotonin receptors tested, i.e. 4-HO-DiPT binds to fewer of the 5-HT receptors compared to psilocin. Klein et al. noted that, “acetylation of the 4-hydroxy group had little effect on HTR potency [in vivo], suggesting that O-acetylated tryptamines may be deacetylated in vivo, acting as prodrugs” of the primary active metabolite, 4-HO-DiPT. In addition to its activity at 5-HT2A, 4-HO-DiPT also interacts with the serotonin transporter (SERT) with IC50 values in the low micromolar range.9
In a 2021 paper, Flanagan et al. studied the structure activity relationship (SAR) of psychedelic compounds (including 4-HO-DiPT) as anti-inflammatory agents using an rat model of asthma.10 They reported that 4-HO-DiPT “does possess full efficacy to normalize PenH [enhanced pause] at 0.5 mg/kg,” demonstrating its effectiveness in eliminating OVA (ovalbumin)-induced lung inflammation in the test animals. In addition, the study examined the effect of the compounds on the in vitro activation of the calcium mobilization signaling pathway (calcium flux) downstream from the 5-HT2A receptor. The authors used the resulting calcium EC50 and Emax data to perform calculations along with PenH-AUC (area under the curve) data to create a proxy measure of the efficacy of anti-inflammatory effects of each compound for calcium mobilization. The results showed “no correlation between canonical signaling [via 5-HT2A Gαq] and anti-asthma efficacy.”
In 2023, Glatfelter et al. reported that 4-HO-DiPT (fumarate and hydrofumarate salt) had submicromolar affinity for 5-HT2A, 5-HT2B, and SERT using cloned human receptors11 (see table at the end). The group also demonstrated that 4-HO-DiPT had low nanomolar affinity for 5-HT1D, Sigma 1 and 2, and M4 receptors. Glatfelter’s data further illustrated the greater selectivity of 4-HO-DiPT compared to other tryptamines, noting that “4-HO-DiPT inhibited binding to fewer 5-HT receptors compared to the other compounds assessed.” Glatfelter et al. also compared the agonist activity of 4-HO-DiPT to other psychedelic tryptamines using serotonin as a baseline within a Gq-calcium mobilization assay. 4-HO-DiPT compound showed a weak potency (EC50 = 6,400 nM) and/or partial agonist efficacy (~72%) at 5-HT2C relative to serotonin and the other structurally similar tryptamines that were tested.
In 2024, Kelly et al. reported that 4-HO-DiPT had strong agonist activity at human 5-HT2A/2B/2C and was almost a full agonist at 5-HT2A.12 The study also reported that “4-OH-DiPT reduced the fear responses (anxiolytic effect) of mice during extinction training in a dose and sex-dependent manner.”
Potential Applications for 4-HO-DiPT and Prodrugs
In 2021, PSR published an article predicting that prodrugs would be an important area of psychedelic drug development. The following year, Malaca et al. published a metabolic study of 4-AcO-DiPT, showing its conversion (via ester hydrolysis) to 4-HO-DiPT.13 Malaca et al. also demonstrated that 4-HO-DiPT is further converted into six metabolites after 3 hours of incubation with human hepatocytes via pathways including O-glucuronidation, O-sulfation, N-oxidation, and N-dealkylation. The N-dealkylation product, 4-HO-NiPT was subsequently prepared and characterized by Laban et al. in 2023.14 In 2024, Sherwood et al. reported cellular pharmacology and HTR data demonstrating that 4-HO-NiPT has psychedelic-like activity – meaning that a least one metabolite of 4-HO-DiPT is also, itself, an active psychedelic compound.15
In 2024 Bryson et al. published the “Synthesis and Activity of a Novel Serotonergic Psychedelic Prodrug of 4-Hydroxy-N,N-diisopropyltryptamine,” reporting 4-O-glutaroyl prodrugs and concluding that “the glutarate prodrug concept is an effective means of introducing the active species of 4-hydroxytryptamines to systemic circulation by injection” and 4-glutarato-DIPT serves as a prodrug of 4-HO-DiPT.5 In support of this conclusion, Bryson et al. performed prodrug cleavage studies, exposing the RE104 prodrug to plasma from various animal species and measuring the concentration of RE104 (prodrug) and 4-OH-DiPT (metabolite) at incubation times of 0, 0.5, 1, 2, and 4 h at 37 °C using a LC tandem MS method. They arrived at the following conclusion: “Taken together, these data confirm the relatively rapid conversion of RE104 [4-glutarato-DIPT] to 4-HO-DiPT in vivo.” The authors did not comment on other metabolites. See Malaca et al. above.
Consistent with Shulgin’s comments in TIHKAL, 4-HO-DiPT has been proposed as a shorter-acting alternative to psilocybin/psilocin in a clinical setting. Clinical use of psilocybin (a prodrug of psilocin) has been criticized due to its 4-8 hour duration, slow onset (taking up to 45 minutes), and an “afterglow” period lasting 4-24 hours.16,17 In a clinical setting, these properties require long periods of observation after administering the drug. Arguably, 4-HO-DiPT (or its prodrugs) could address these concerns, lessening onset time and shortening duration in treatment settings.
The altered time course of 4-HO-DiPT’s effects could produce varying therapeutic effects in comparison to the longer lasting psilocybin. It is currently unclear from the literature and clinical trials whether the duration of psychedelic effects is an important factor in treatment of underlying conditions. Hopefully, further research will clarify which pharmacokinetic parameters are required for maximum therapeutic efficacy (Tmax, Cmax, AUC, etc.).
Scientists have described the unmet need for high purity (i.e. crystalline), well-characterized tryptamine compounds as one of the impediments to progress in downstream biological studies.19 In short, failing to accurately characterize a molecule introduces ambiguity and error to all downstream data because errors in determining the composition and molecular mass affect subsequent calculations such as concentration, potency, etc.. Given the potential for tryptamine compounds to exist in multiple crystalline forms, including various solvates, salts, and (in the case of psilocybin or diacid “hemiesters” like 4-glutarato-DiPT) zwitterions, it is especially important to rigorously characterize these compounds to ensure the correct chemical composition for accurate dosing. As Dr. Alexander Sherwood of the Usona Institute explained, “Crystal structures are the gold standard in chemical characterization.”20
On January 14, 2022, the US Drug Enforcement Administration proposed placing 4-HO-DiPT in Schedule I of the Controlled Substances Act.21 However, due to an effective public response, the agency withdrew the proposal in July 2022.22
Dose Data
| Dose (mg/kg) | Administration | Species | Ref. | |
|---|---|---|---|---|
| ED50 | 1.03 | Intraperiotoneal | Mouse | 8 |
Receptor Binding Affinity Data
| Receptor | Ki (nM) | Species | Note | Ref. |
|---|---|---|---|---|
| 5-HT1A | 5700 | Human | 9 | |
| 5-HT1D | 1860 | Human | 11 | |
| 5-HT2A | 728 | Human | 9 | |
| 5-HT2A | 922 | Human | 11 | |
| 5-HT2B | 460 | Human | 9 | |
| 5-HT2B | 85 | Human | 11 | |
| 5-HT2C | 2800 | Human | 9 | |
| SERT | 1800 | Human | 9 | |
| SERT | 816 | Human | 11 | |
| M4 | 1725 | Human | 11 | |
| Sigma 1 | 1063 | Human | 11 | |
| Sigma 2 | 2215 | Human | 11 | |
| H1 | 9800 | Human | 9 |