In a 2019 paper published in Nature Scientific Reports, researchers announced the discovery of a novel cannabinoid isolated from Cannabis sativa L.1 The compound, (-)-trans-Δ9-THCP (Δ9-tetrahydrocannabiphorol), has the same chemical structure as (-)-trans-Δ9-THC (Δ9-tetrahydrocannabinol) but with a seven-term alkyl side chain (Figure 1).
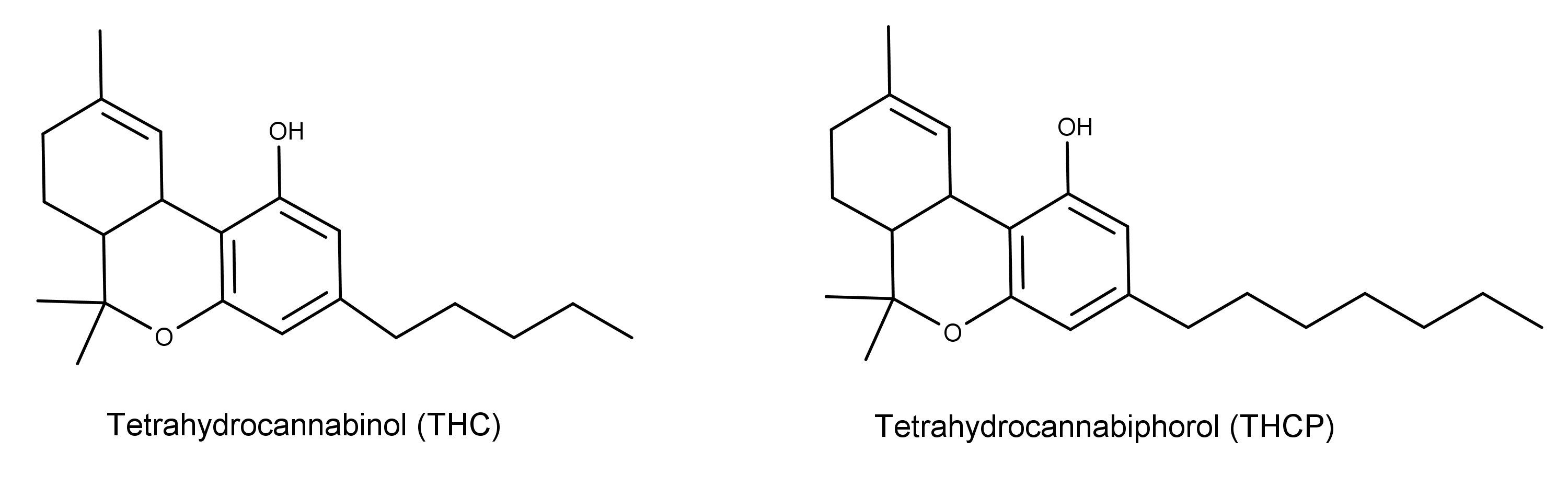
Figure 1: The chemical structures of Δ9-THC and the newly-isolated Δ9-THCP. Note the seven carbon side chain on THCP compared to the five carbon side chain on THC.
The Length of the Side Chain is Key to the Effects of Δ9-THC Analogs
Previous studies indicate that synthetic Δ9-THC analogs with longer side chains have more significant pharmacological effects than Δ9-THC at CB1 (cannabinoid receptor type 1).2 At least three carbons are required in the side chain to bind to CB1. Receptor binding activity increases up to the point there are eight carbons in the side chain. Beyond eight carbons, the binding activity decreases. Until this current study by Citti et al., the five-carbon side chain of Δ9-THC was the longest naturally-occurring side chain known in these analogs.
Δ9-THCP Has Greater Receptor Binding Activity
In in vitro testing using radioligand binding, the data indicated that binding activity (Ki) of Δ9-THCP at the human CB1 and CB2 receptors was 1.2 nM and 6.2 nM, respectively. The authors performed the same receptor testing on the analogs (-)-trans-Δ9-THC, (-)-trans-Δ9-THCB (tetrahydrocannabutol), and (-)-trans-Δ9-THCV (tetrahydrocannabivarin). Table 1 below summarizes the results (Read more about Ki values and what they mean in the PSR article Binding of Psilocin and Psilocybin to Serotonin Receptors).
Table 1: Ki values for Δ9-THCP and analogs. Table reproduced from Citti et al. 2019.
| Compound | R Group | hCB1 Ki (nM) | hCB2 Ki (nM) |
|---|---|---|---|
| (-)-trans-Δ9-THCV | -propyl | 75.4 | 62.8 |
| (-)-trans-Δ9-THCB | -butyl | 15.0 | 51.0 |
| (-)-trans-Δ9-THC | -pentyl | 40.0 | 36.0 |
| (-)-trans-Δ9-THCP | -heptyl | 1.2 | 6.2 |
Using Δ9-THCP in the cannabinoid tetrad pharmacological test in mice, the researchers observed hypomotility, analgesia, catalepsy, and decreased rectal temperature. All of these effects are understood to be physiological manifestations of cannabinoid activity.
There is Much More to Learn About the Entourage Effect
This study drives home the importance of identifying and understanding all of the compounds in naturally-occurring organisms. On its own, each Δ9-THC analog has its unique effects on cannabinoid receptors. But, more than one analog, and even more in certain combinations (and differing amounts), results in different effects.
The discovery of naturally-occurring Δ9-THCP in Cannabis sativa adds to the complexity of understanding of the entourage effect in cannabis. As the authors of this paper keenly observed:
The presence of this new phyrocannbinoid could account for the pharmacological properties of some cannabis varieties difficult to explain by the presence of the sole Δ9-THC.