
The major psychoactive compound Δ9-Tetrahydrocannabinol (THC, Figure 1) present in the plant Cannabis is characterised by its analgesic and mood-enhancing effects.1 Cannabis has been used both recreationally and medically from the early ages of human history.2 With its good safety profile several THC-based compounds are approved for therapeutic use as countries have legalised the drug.1,3
Synthetic Cannabinoids
Contrary to THC, synthetic cannabinoids are a group of compounds designed to mimic the effects of cannabis. Commonly referred to as K2 or Spice, these synthetic compounds have gained notorious attention as dangerous substitutes. These compounds have been known to induce a ‘zombie-like’ state in users and have been attributed to several hospitalization and clusters of fatalities.4,5
MDMB-Fubinaca, chemical name methyl (S)-2-(1-(4-fluorobenzyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate, (Figure 1), a synthetic indazole cannabinoid, has been assigned one the deadliest synthetic cannabinoids being 20-fold more potent than THC.6
As illustrated in Figure 1, the chemical structure of THC consists of a tricyclic scaffold containing a pyran and phenolic ring with a pentyl side chain. MDMB-Fubinaca consists of a core indazole ring, a p-Fluorobenzyl, and an amino acid side chain with a methylated ester group. Despite the diverse structural difference between THC and MDMB-Fubinaca, both compounds bind to and activate the cannabinoids 1 receptor (CB1).
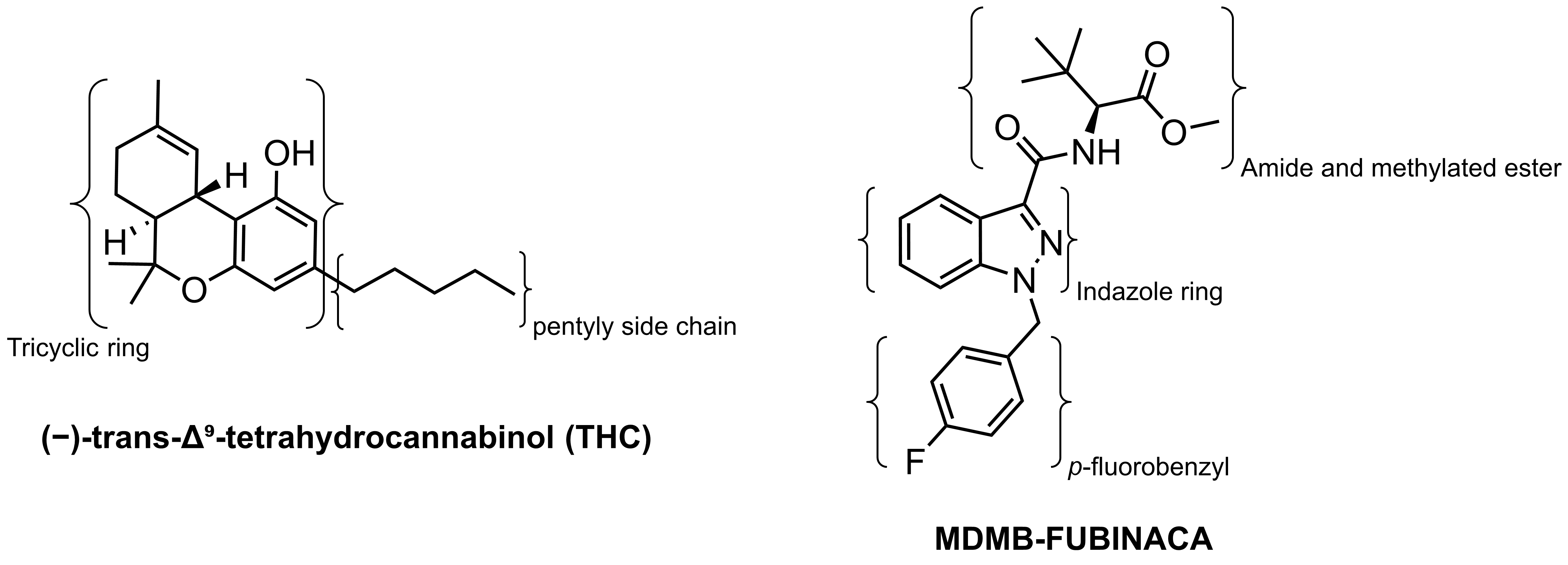
Figure 1: (click to enlarge) The chemical structures of THC and MDMB-Fubinaca. Functional groups are labelled for clarity. Image created by Ian Liddle.
Cannabinoid Receptor Activation
CB1 is a type of G protein-coupled receptor (GPCR) a large protein family characterised by their seven transmembrane structures that functions to transduce and amplify external signals to activate signalling pathways within the cell.
Both THC and MDMB-Fubinaca act as agonists that bind and activate CB1. However, THC is only a partial agonist able to bind to CB1 but unable to induce a maximal response.7 While MDMB-Fubinaca binds and acts as a full agonist inducing a maximal response for greater activation.8 The increased activation of CB1 by MDMB-Fubinaca and other synthetic cannabinoids gives them a greater potency and is attributed to their side effects and lethality.
How does MDMB-Fubinaca increase CB1 activation?
The difference in the partial versus full agonist effect of these compounds is attributed to their different chemical structures (Figure 1). The different chemical structures make them able to form different binding interactions with CB1. Kumar et al. crystallised CB1 with bound MDMB-Fubinaca to gain an understanding of the molecular interaction contributing to the increased potency.9
The CB1 and MDMB-Fubinaca crystal structure is shown in Figures 2 and 3. The characteristic seven-transmembrane structure of GPCRs is present as MDMB-Fubinaca binds at the active site. As Kumar et al. described, MDMB-Fubinaca occupies a C-shape geometry to form significant hydrophobic interactions with CB1 to maintain a rigid shape.
The authors go on to say that the p-fluorobenzyl group forms π-π interactions with a Tryptophan (Trp) residue Trp279, whilst the amino acid side chain forms polar interactions with Histidine (His) His178 and the tert-butyl group forms additional hydrophobic interaction to maintain the C-shape orientation. Most notably is the interaction between the indazole core with Phenylalanine (Phe) Phe200 and Trp356 of CB1. These two residues, Phe200 and Trp356, are part of a ‘twin-toggle switch’ which activates CB1. The hydrophobic interactions between the indazole core Phe200 and Trp356 lock the CB1 receptor into an active position able to maintain an ‘on’ status resulting in increased potency.
These strong interactions between Phe200, Trp356, and the receptor are hypothesised to be absent in THC binding to CB1. Whilst no crystal structure of THC bound to CB1 exists, similar structural analogues are available. It has been strongly suggested that the greater flexibility of THC contributes to its decreased potency and better safety profile.9 For instance, in THC, the flexible pentyl sidechain fluctuates between the position occupied by the p-fluorobenzyl group and the interactions with the ‘twin-toggle switch’. Therefore, THC is unable to constantly maintain an ‘on’ position due to the increased flexibility reducing its potency.
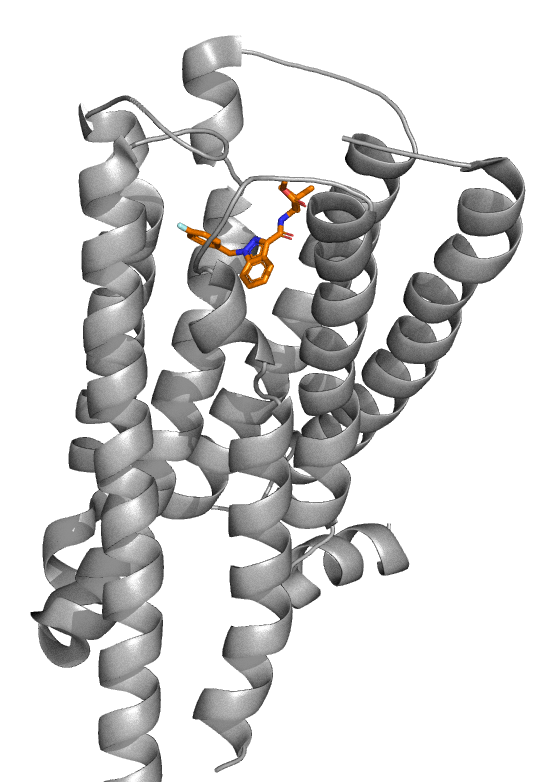
Figure 2: Cartoon structure of CB1 (RCSB PDB: 6N4B, grey ribbon) with MDMB-Fubinaca (orange carbons) bound. Image adapted from Kumar et al. and PDB: 6N4B, and created by Ian Liddle.

Figure 3: MDMB-Fubinaca interactions with CB1 binding site. The main interacting residues are shown in green with the ‘twin-toggle switch’ residues in cyan. CB1 is locked into an ‘on’ position upon MDMB-Fubinaca binding. Image adapted from Kumar et al. and RCSB PDB: 6N4B, and created by Ian Liddle.
The MDMB-Fubinaca Structure Allows for Safer Drug Design.
Kumar et al. have demonstrated the potency of the synthetic cannabinoid MDMB-Fubinaca compared to THC on a structural basis. Notably, MDMB-Fubinaca forms increased hydrophobic interactions with the ‘twin-toggle switch’ residues Phe200 and Trp356 to maintain the receptor in the ‘on’ position.
The difference between activation by the partial agonist THC and the full agonist MDMB-Fubinaca is an important consideration in designing safer drugs. This crystal structure gives researchers a molecular insight into the activation mechanism of CB1 and the structural differences between lethal and non-lethal drugs. With several drug candidates in clinical trials, understanding the molecular difference between a full and partial agonist can allow the design of safer cannabinoid-based therapeutics.
Hi! Thanks for the article. I work in this field and wanted to make a correction. Firstly, this is not a crystal structure. This structure is actually of the whole CB1/G protein complex and was obtained by cryogenic electron microscopy (cryo-EM). Because G protein coupled receptors are only fully active in presence of the G proteins, this is a big advantage of this methodology (cryo-EM) for understanding how drug binding can influence activation states (e.g. full vs. partial agonism) as it’s closer to physiology. I think you gave a very nice summary of one of the main hypotheses for THC… Read more »
Hi Kieran, thank you for the comment. You are indeed correct this is not a crystal structure and it is a cryo-EM structure. If I were to re-write this article it would be a major correction for me to fix.
Since I wrote this blog there have several aritcle using molecular dynamic simulations that suggest THC does likley stabilise as ‘partial’ active state, which is through the interaction with Trp356. (See: doi.org/10.1038/s41598-021-01767-5 ; doi.org/10.1021/acschemneuro.0c00547 and doi.org/10.3389/fmolb.2022.860035).
Were you successful with your THC-CB1R-G protein cryo-EM structure?
~Ian L