Nature has equipped organisms with a variety of receptors for interacting with their environment. One type of receptor, the G protein-coupled receptor (GPCR), is of particular interest in psychedelic research. The serotonin 5-HT2A receptor, which elicits the psychedelic effect, belongs to the GPCR family. Also, research is revealing the unique role that GPCRs may play when it comes to targeting drugs.
GPCR Basics
The GPCRs are a large and diverse family of proteins. They comprise around 800 members, creating the single largest class of membrane receptors in humans.2 The main function of GPCRs is transducing external stimuli into signals which are then propagated into cells. Inside the cell, the transmitted signal activates signal transduction pathways, resulting in a biological response.
GPCRs respond to a variety of stimuli and activate signaling pathways involving neurotransmitters and hormones.3 They are also mediators for stimuli involving taste, vision, and smell.
GPCRs have a characteristic structure consisting of seven helices that span the cell membrane (Figure 1). Their structure was first resolved in the 1990s using two-dimensional crystals of the rhodopsin.4,5 In 2000, Palczewski et al. published the first crystal structure of bovine rhodopsin.6 The G protein part of the GPCR comes from the fact that each receptor is coupled to a G protein (guanine nucleotide-binding protein) molecule inside the cell.2
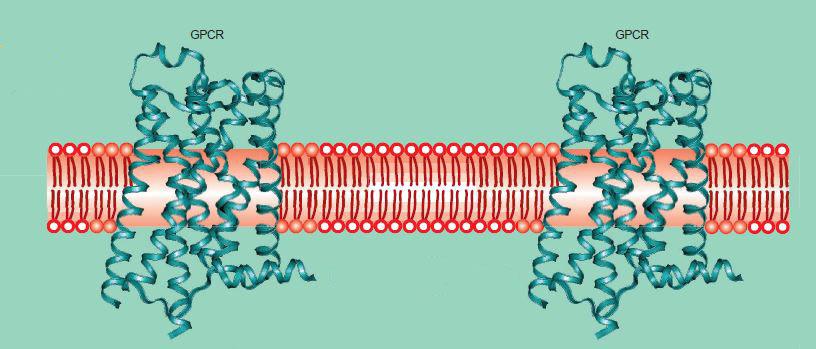
Figure 1: An illustration showing how the seven helices of GPCRs span the cell membrane.7
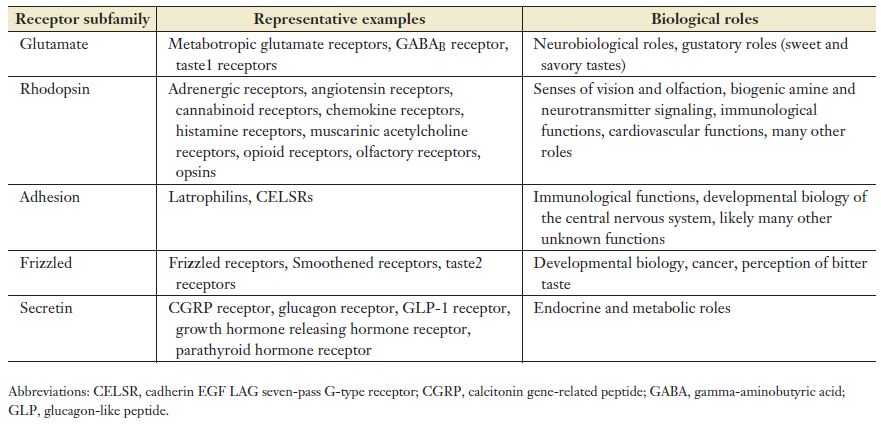
There are five families of GPCRs based on their structural similarities and DNA sequence.8,9 The families form an acronym known as GRAFS: glutamate, rhodopsin, adhesion, frizzled/taste2, and secretin. The rhodopsin family is the largest of the five. The table below, taken from Erlandson et al., lists some examples and functions of the receptors in the GPCR subfamilies.2
The commonalities of GPCRs stand in contrast to the breadth and complexity of their functioning.3
…individual GPCRs have unique combinations of signal-transduction activities involving multiple G-protein subtypes, as well as G-protein-independent signalling pathways and complex regulatory processes.
How GPCRs Work
As discussed earlier, signal transduction in cells is the primary function of GPCRs. The mechanisms of signal transduction are many, and some get quite complex. Briefly, when an agonist binds, the receptor changes conformation (shape).2 The change in shape activates and causes a “dramatic structural rearrangement” in the conformation of the G protein.
There are several types of G proteins, so the downstream signaling pathways from this point depend on which one is coupled to the receptor.10,11 Some of the most well studied GPCR signaling events are the stimulation of the enzyme phospholipase C and the stimulation and inhibition of adenylyl cyclase.12 The signaling initiated by GPCRs is dampened by the action of proteins called arrestins binding to the receptor.13
There is research indicating that single GPCRs are not acting alone or by themselves in producing their effects. In particular, oligomerization of the receptor may play a central role in its activity14-17 (for example, see the rhodopsin receptor complex pictured at the beginning of this article). In a 2002 review paper, George et al. recognized the additional complexity that the concept of oligomerization brings to the study of GPCRs.14
For the future, perhaps the greatest challenge facing the pharmaceutical industry will be to integrate GPCR homo- and hetero-oligomerization (as well as GPCR interactions with other proteins) into the molecular models that are used in the development of novel and improved therapeutics.
Allosteric Modulation
Understanding the phenomenon of allosteric modulation (AM) is essential for getting the full picture of GPCR functioning. The mechanics of AM sound simple. Basically, a ligand binds at a site (the allosteric site) other than the orthosteric (primary) binding site.18 After the AM binds, things get a little more complicated.
The binding of AMs causes conformational changes in the receptor and other alterations in the receptor state. These alterations change in how the receptor interacts with the endogenous ligand (the molecule that binds to the orthosteric site), an agonist, or antagonist. There are positive AMs (PAMs) that increase the affinity, efficacy, or both of the receptor for the endogenous ligand. Negative AMs (NAMs) decrease these properties.
In their 2018 paper, Erlandson et al. called GPCRs “complex allosteric machines.” 2 The authors state that GPCRs “…are networks of allosterically linked structural switches.” They explain that only does AM affect the GPCRs themselves, but also other molecules like the G proteins and arrestins.
In 2009, Conn et al. stated the following regarding allosteric compounds of GPCRs that have been developed,19
These compounds provide high selectivity, novel modes of efficacy, and may lead to novel therapeutic agents for the treatment of multiple psychiatric and neurological human disorders.
Targeting allosteric sites on GPCRs presents many opportunities for drug exploration. Although AM is not unique to GPCRs, they are by far the largest class of targets for modern-day drugs.14 When it comes to the entourage effect in naturally occurring psychedelic cocktails, this adds another deep layer of complexity and mystery for scientific study.
More Research is Needed on GPCRs and Psychedelics
GPCRs are intimately involved in the psychedelic effect. However, scientists still don’t understand how the entourage effect may influence the effects of nature’s psychedelic cocktails. On top of that, the likelihood of AM also influencing the effects of psychedelics at GPCRs is creating more research opportunities.
For those interested in all things GPCR, check out the GPCR database maintained by the University of Copenhagen.