
The compounds aeruginascin and bufotenidine come from two different taxonomic kingdoms, Plantae and Animalia, respectively. This makes them seem quite different, but the compounds may be related in other ways. This similarity is evidence of common origins and the interconnectedness of living things on Earth. As discussed below, the similarities and differences between aeruginascin and bufotenidine bring insight and many opportunities for psychedelic researchers.
Aeruginascin is one of several compounds found in magic mushrooms (aka psilocybin mushrooms) but so far has only been found in the species Inocybe aeruginascens.1 Bufotenidine (not to be confused with bufotenin) is one compound in the secretions of certain toad species like Bufo bufo gargarizans.2 Interestingly, in 2013, Servillo et al. detected bufotenidine in plants of the genus Citrus.3
Comparing the Chemical Structures of Aeruginascin and Bufotenidine
The chemical structure of aeruginascin is similar to psilocybin (see below), the primary psychedelic prodrug in magic mushrooms. The difference is that aeruginascin has three methyl groups on the ethanolamine moiety, and psilocybin has two. The additional methyl group creates a positively charged trimethylammonium group on aeruginascin.4
The difference between aeruginascin and bufotenidine is that bufotenidine is dephosphorylated aeruginascin with the HO- group at the 5-position instead of the 4-position. Also, notice that bufotenidine has a charged trimethylammonium group like aeruginascin.
Comparing the Pharmacology of Aeruginascin and Bufotenidine
In 2004, Jensen hypothesized that aeruginascin is unlikely to cross the blood-brain barrier due to its quaternary ammonium group.4 Because of this barrier, he stated that aeruginascin might have “profound peripheral effects.”
By analogy to Psilocybe alkaloids, Jensen also hypothesized that aeruginascin is metabolized quickly by the removal of the phosphate group. It’s dephosphorylation product, 4-HO-TMT, is structurally similar to bufotenidine except for the difference in the -OH substitution in the 4th/5th position. Bufotenidine is a potent and selective agonist of the serotonin 5-HT3 receptor.5 It binds to 5-HT3 receptor with ten times the affinity of serotonin.6
5-HT3 is unique in the serotonin receptor family. It is not a G protein-coupled receptor (GPCR). It works via a ligand-gated ion channel.7 One of the effects of activation of the 5-HT3 receptor is nausea and vomiting which are common side effects of chemotherapy and radiation treatments. Antagonist drugs help control these side effects in cancer patients.8
A 2017 radioligand binding study found that bufotenidine from the parotid gland of the toad Bufo bufo had a higher affinity for neuronal α7 nicotinic acetylcholine receptors compared with muscular cholinergic receptors.9 A study from the early 1970s indicated that bufotenidine isolated from the giant reed Arundo donax caused paralysis in rats.10
In a 2020 paper, Sherwood et al. discussed the potential importance of aeruginascin and/or its active metabolite 4-HO-TMT in the condition known as wood lover paralysis (WLP).11 However, the authors did not report any findings regarding the biological activity of either compound.
By putting the chemical and pharmacological pieces together, one can hypothesize how aeruginascin could be responsible for WLP via bufotenidine.
If aeruginascin is dephosphorylated in the body and carbon 4 receives a hydroxyl group as a result, then the only difference between the paralysis-causing bufotenidine and the WLP-causing candidate aeruginascin is whether carbon 4 or 5 has the hydroxyl group. Both molecules have the charged and reactive trimethylammonium group. Therefore, it is feasible they may affect the same receptor and cause the same effect.
In addition, virtually nothing is known about the potential relevance of aeruginascin and bufotenidine to the entourage effect. That understanding would allow researchers to make formulations that preserve the benefits of nature’s cocktails while also having pharma’s precise dosing.
Listening to What Nature is Saying
Comparing naturally occurring compounds reveals hints from nature to apply to research for answering fundamental questions. Although aeruginascin and bufotenidine come from different taxonomic kingdoms, they are related chemically and also possibly in their pharmacology.
Aeruginascin (and a few other magic mushroom compounds) is now receiving research attention thanks to the work of Sherwood et al. However, bufotenidine pretty much remains in the shadows. There is clearly an unmet need for continuing research into aeruginascin, bufotenidine, and their metabolites.
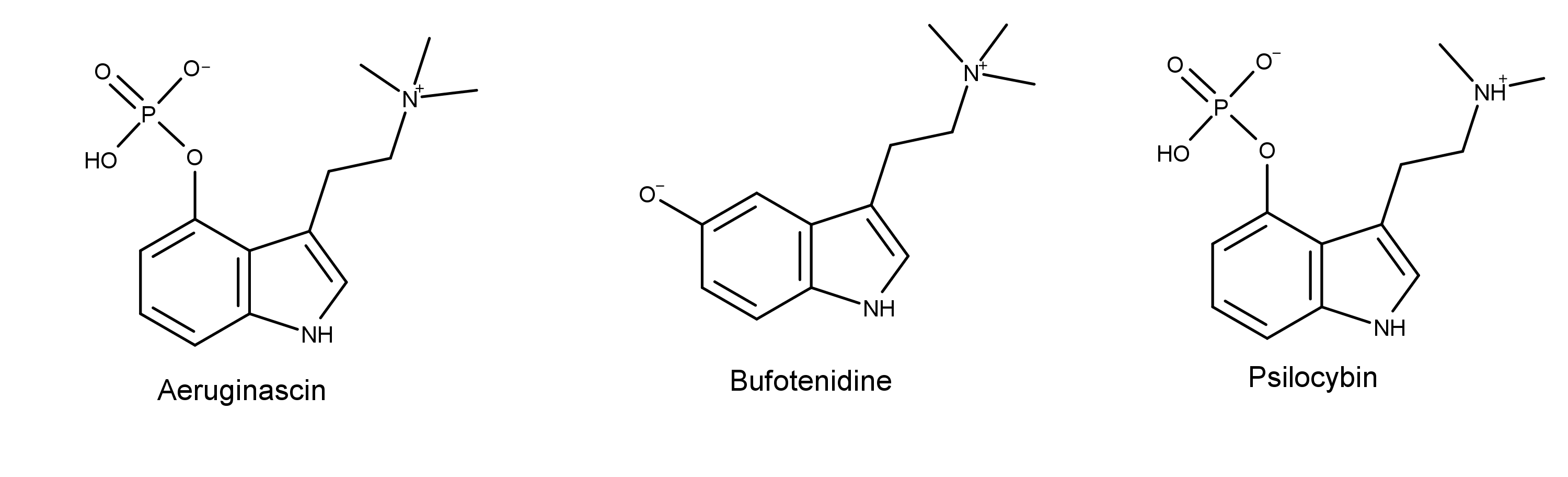

“…Jensen also hypothesized that aeruginascin is metabolized quickly by the removal of the phosphate group. It’s dephosphorylation product, bufotenidine, is a potent and selective agonist of the serotonin 5-HT3 receptor.” Bufotenidine is not the dephosphorylation product of aeruginascin. Bufotenidine has the 5 hydroxyl whereas the product of aeruginascin dephosphorylation has the 4 hydroxyl.
Hi Wilson, thanks for letting us know about that error. I made the correction.