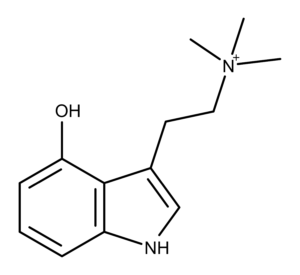
4-HO-TMT is the putative metabolic product of the magic mushroom compound aeruginascin.1 It is also an analog of the most well-known magic mushroom compound, psilocybin.
The Chemistry of 4-HO-TMT
4-HO-TMT is aeruginascin without the phosphate group on carbon 4. According to a 2006 study by Jensen et al., 4-HO-TMT gave an immediate gray color with Ehrlich’s reagent compared to aeruginascin which was purple.1 In their thin-layer chromatography (TLC) tests, the researchers also found that 4-HO-TMT had an Rf of 0.62 using a n-PrOH/NH3 28% eluent.
In July 2020, researchers from CaaMTech, the University of Massachusetts Dartmouth, and Canopy Growth USA published a synthesis method for 4-HO-TMT using 4-AcO-DMT (psilacetin) as the starting material.3 Here are the synthesis steps:
- 4-AcO-DMT fumarate is methylated in the presence of excess iodomethane, resulting in 4-AcO-TMT (4-acetoxy-N,N,N-trimethyltryptammonium) iodide. Chadeayne et al. call this compound a functional analog of aeruginascin.
- 4-AcO-TMT iodide is hydrolyzed to 4-HO-TMT iodide in aqueous acetic acid.
- 4-HO-TMT iodide is purified by recrystallization in a methanolic solution.
- Both 4-HO-TMT and 4-AcO-TMT iodide salts were recrystallized from water to obtain their single-crystalline forms.
The Chadeayne et al. research team confirmed the identity and high purity of the compounds by using NMR and elemental analysis. Further, they solved the crystal structures of the iodide salts of 4-HO-TMT and 4-AcO-TMT.
The authors reported that “These are the first two quaternary tryptammonium salts ever characterized by single-crystal diffraction.”
The Pharmacology of 4-HO-TMT
Up until the paper published by Chadeyane et al. in 2020, there was no research on the pharmacology of 4-HO-TMT. The research team tested the binding affinity of 4-HO-TMT and 4-AcO-TMT at four serotonin receptors, 5-HT1A, 5-HT2A, 5-HT2B, and 5-HT3. The table at the end of this article shows the receptor testing data. For more information on receptor binding values and what they mean, see the PSR article Binding of Psilocin and Psilocybin to Serotonin Receptors.
4-AcO-TMT showed no binding activity at any of the receptors tested. However, 4-HO-TMT had activity at 5-HT1A, 5-HT2A, and 5-HT2B. Agonist activity at 5-HT2A is regarded by experts as the hallmark of psychedelic activity.4
Although 4-AcO-TMT showed less binding affinity at 5-HT2A than psilocin, the authors concluded that the Ki values were comparable (670.0 nM and 107.2 nM, respectively). Also, the data showed that 4-HO-TMT has a similar affinity for 5-HT2B compared to psilocybin.
One hypothesis tested by Chadeayne et al. was that aeruginascin’s active metabolite 4-HO-TMT would have an affinity for 5-HT3 like bufotenine, a structurally similar tryptamine.1 The data showed this was not the case. The Ki for 4-HO-TMT at 5-HT3 was >10,000 nM.
The authors also noted an important observation regarding the activity of 4-HO-TMT at the 5-HT2B receptor. In 2000, Rothman et al. proposed the involvement of 5-HT2B receptor activation with valvular heart disease (VHD).5 In the Chadeayne et al. study, the data indicated that 4-HO-TMT had significantly less binding affinity for 5-HT2B than psilocin. The comparison of Ki values is considered state of the art for estimating the safety of a compound in relation to 5-HT2B and VHD.6
Despite the value gained from these tests, it is unclear why Chadeayne et al. did not test the two compounds at nicotinic acetylcholine receptors. The results may have shed some light on the possible role of aeruginascin (or its metabolite) in the phenomenon known as wood lover paralysis.
The Applications and Potential of 4-HO-TMT
The research work done by Chadeayne et al. has made several significant contributions and opened up a new area for psychedelic compound research. The surprising results showing the absence of activity at the serotonin 5-HT3 receptor, and the presence of significant activity at 5-HT2A, presents some intriguing questions.
For example, Chadeayne et al. say that by having a quaternary ammonium group, it appears unlikely that 4-HO-TMT could cross the blood-brain barrier (BBB) and directly affect 5-HT2A receptors in the brain. However, they point to a 2014 study indicating that another quaternary ammonium compound crosses the BBB.7 In this study, Zacny et al. showed that the compound known as methylnaltrexone or MTNX, crossed the BBB in humans, possibly via cellular transporters. Chadeayne et al. concluded, “therefore, psychotropic activity [of 4-HO-TMT] remains a possibility.”
Receptor Binding Affinity Data
| Receptor | Ki (nM) | Species | Note | Ref. |
|---|---|---|---|---|
| 5-HT1A | 4400 | Human | 3 | |
| 5-HT2A | 670 | Human | 3 | |
| 5-HT2B | 120 | Human | 3 | |
| 5-HT3 | >10000 | Human | 3 |