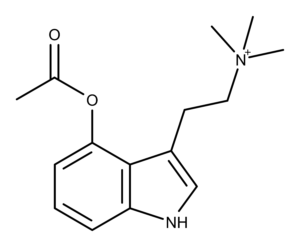
4-AcO-TMT is a functional analog of the magic mushroom compound aeruginascin and the intermediate in a synthesis method for making its purported metabolite 4-HO-TMT.1,2
The Chemistry of 4-AcO-TMT
In July 2020, Chadeayne et al. published a synthesis method for 4-HO-TMT starting with 4-AcO-DMT (psilacetin) fumarate and creating 4-AcO-TMT is an intermediate.1
- 4-AcO-DMT fumarate is methylated in the presence of excess iodomethane, resulting in 4-AcO-TMT (4-acetoxy-N,N,N-trimethyltryptammonium) iodide. Chadeayne et al. call this compound a functional analog of aeruginascin.
- 4-AcO-TMT iodide is hydrolyzed to 4-HO-TMT iodide in aqueous acetic acid.
- 4-HO-TMT iodide is purified by recrystallization in a methanolic solution.
- Both 4-HO-TMT and 4-AcO-TMT iodide salts were recrystallized from water to obtain their single-crystalline forms.
Chadeayne et al. confirmed the identity and high purity of 4-AcO-TMT and 4-HO-TMT by using NMR and elemental analysis. Further, they solved the crystal structures of the iodide salts of 4-HO-TMT and 4-AcO-TMT. The authors stated that “These are the first two quaternary tryptammonium salts ever characterized by single-crystal diffraction.”
The Pharmacology of 4-AcO-TMT
Up until the paper published by Chadeyane et al. in 2020, there was no research on the pharmacology of 4-AcO-TMT. The research team tested the binding affinity 4-AcO-TMT (and 4-HO-TMT) at four serotonin receptors, 5-HT1A, 5-HT2A, 5-HT2B, and 5-HT3. The table at the end of this article shows the receptor testing data. For more information on receptor binding values and what they mean, see the PSR article Binding of Psilocin and Psilocybin to Serotonin Receptors. As the data show, 4-AcO-TMT showed no binding activity at any of the receptors tested.
The Applications and Potential of 4-AcO-TMT
The research work done by Chadeayne et al. has made several significant contributions and opened up new areas for researching psychedelic compounds. Despite the value gained from this work, it is unclear why Chadeayne et al. did not test 4-AcO-TMT at nicotinic acetylcholine receptors. The results may have shed some light on the possible role of aeruginascin (or its metabolite) in the phenomenon known as wood lover paralysis.
Receptor Binding Affinity Data
| Receptor | Ki (nM) | Species | Note | Ref. |
|---|---|---|---|---|
| 5-HT1A | >10000 | Human | 1 | |
| 5-HT2A | >10000 | Human | 1 | |
| 5-HT2B | >10000 | Human | 1 | |
| 5-HT3 | >10000 | Human | 1 |