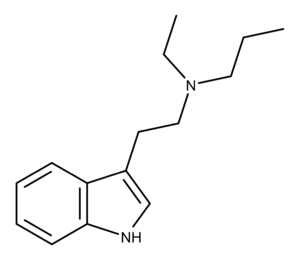
EPT is an unsubstituted tryptamine compound. Structurally, it is the N-ethyl analog of DPT (N,N-dipropyltryptamine) and the N-propyl analog of DET (N,N-diethyltryptamine). There is no entry for EPT in Alexander and Ann Shulgin’s book TiHKAL.1
The Chemistry of EPT
Until recently, there was only scattered mention of EPT in the scientific literature.2–4 In 2020, Chadeayne et al. published the crystal structure of the fumarate salts of EPT and another DMT analog, MALT (N-methyl-N-allyltryptamine).5 The authors explain that their reasoning for studying these two compounds centers on understanding the structure-activity relationships between similar compounds, as well as their “cooperative biological activity.” They added that “The preparation of pure crystalline forms of these compounds is essential to conducting meaningful biological studies and ultimately developing drug products.”
The Pharmacology of EPT
Although nothing is known about the pharmacology of EPT, based solely on its chemical structure, it may be an agonist at the serotonin 5-HT2A receptor.
The Applications and Potential of EPT
Studying the applications and potential for EPT is a wide-open area for research.