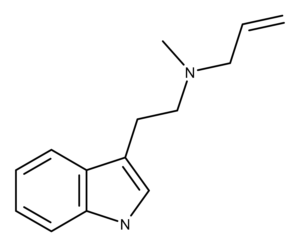
MALT is an obscure, synthetic, unsubstituted tryptamine compound. There is no entry for EPT in Alexander and Ann Shulgin’s book TiHKAL.1
The Chemistry of MALT
Until recently, there was only scattered mention of MALT in the scientific literature.2–4 In 2020, Chadeayne et al. published the crystal structure of the fumarate salts of MALT and another DMT analog, EPT (ethylpropyltryptamine).5 The authors explain that their reasoning for studying these two compounds centers on understanding the structure-activity relationships between similar compounds, as well as their “cooperative biological activity.” They added that “The preparation of pure crystalline forms of these compounds is essential to conducting meaningful biological studies and ultimately developing drug products.”
The Pharmacology of MALT
Although nothing is known about the pharmacology of MALT, based solely on its chemical structure, it may be an agonist at the serotonin 5-HT2A receptor.
The Applications and Potential of MALT
Studying the applications and potential of MALT is a wide-open area for research.