The Pharmacology of Noribogaine
Noribogaine is a metabolite of the compound ibogaine that comes from the plant Tabernanthe iboga (aka iboga). Noribogaine was first identified as the primary metabolite of ibogaine by Deborah Mash et al. in 1995.1 This study also found that noribogaine caused “marked dose-related elevation of extracellular 5-HT [serotonin].” The authors hypothesized that the increased levels of serotonin in the brain might elevate a person’s mood. In turn, this may explain the ability of Iboga (via ibogaine) to damper drug cravings. Noribogaine did not, however, elevate the levels of dopamine in the nucleus accumbens in the brain over the dose range they tested.
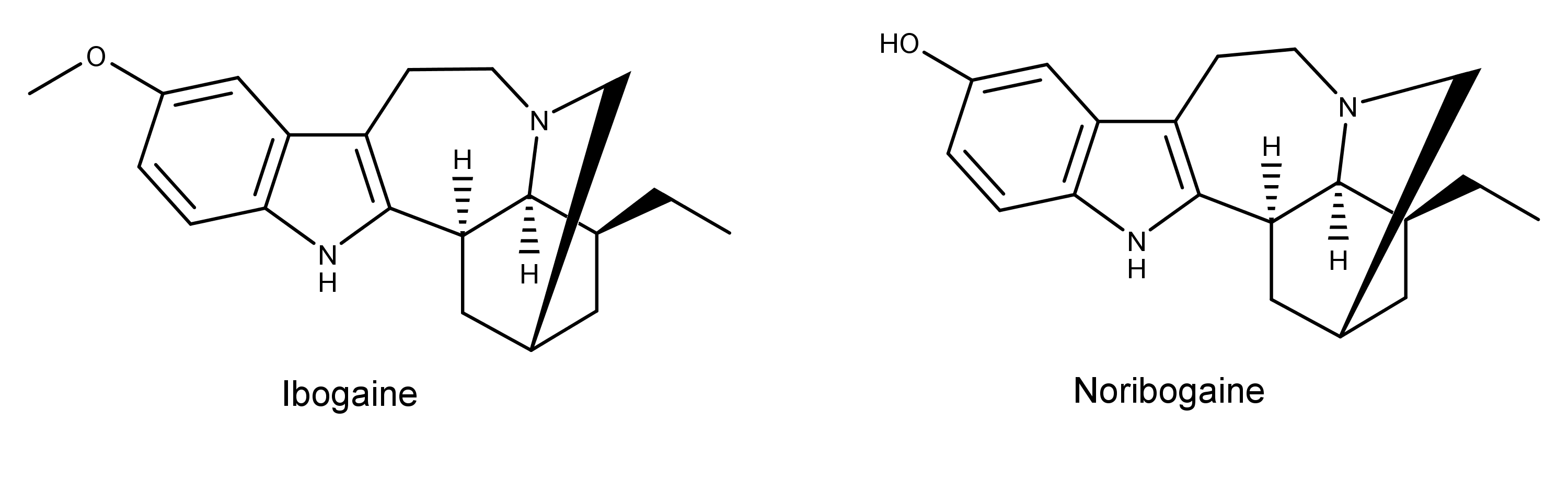
In 1998, researchers discovered that the liver enzyme cytochrome P4502D6 catalyzes the O-demethylation of ibogaine to noribogaine.2
Noribogaine remains in the circulation for several days and may be responsible for many of its effects in humans.3 A 1997 study by Pearl et al. observed that male and female rats had similar levels of noribogaine in their brains 19 hours after peritoneal injection.4 However, their plasma levels of noribogaine were 10-20 fold less. From this, the authors theorized that noribogaine crossed the blood-brain barrier.
In 1998, Pablo and Mash observed that noribogaine was a full agonist at the rat mu opioid receptor.5 These results were in contrast to ibogaine, which had no significant effect. From this, the authors hypothesized,
The efficacy of noribogaine as a full μ-opioid agonist may explain ibogaine’s ability to block the acute signs of opiate withdrawal and its suppressive effects on morphine self-administration.
A 2006 study by Baumann et al. found that noribogaine was less likely to produce tremors and forepaw treading in rats compared to ibogaine.6 The authors stated that this feature of noribogaine might make it a safer alternative for drug development.
A 2008 study found the median lethal dose (LD50) of noribogaine was 630 mg/kg in mice.7 The study found the toxicity of ibogaine was about 2.4 times higher than noribogaine (263 mg/kg).
In 2015, Maillet et al. showed that noribogaine is a G-protein biased kappa opioid receptor agonist.8 Noribogaine’s receptor affinity was Ki = 720 nM in this study. This indicates it has a greater affinity for the receptor than ibogaine, which has a Ki = 2,600 – 3,680 nM.8,9
The Applications and Potential of Noribogaine
Building on the 1998 work of Pablo and Mash and others, there is a growing body of research indicating that noribogaine possesses anti-addictive properties. For example, noribogaine decreased morphine and cocaine self-administration in animal studies.9,10 Other studies have shown that in rats, noribogaine decreased ethanol11 and nicotine12 self-administration.
In a 2020 study using rats, Rodríguez et al. found that a single dose of noribogaine (or ibogaine) caused antidepressant-like effects.13
Over the years, ibogaine has developed a stigma due to its potential cardiac effects.14,15 As a result, studying noribogaine has been met with similar hesitancy. Notably, a 2017 case study suggested: “…noribogaine was associated with life-threatening cardiac arrhythmias due to its prolonged presence, while ibogaine plasma-levels were very low.” 16 It should be noted that the patient in this case study had ingested internet-purchased capsules allegedly containing ibogaine. The authors of the case study say the patient ingested 1400 mg of the product over 12 hours. In contrast, a 2015 study found oral doses of noribogaine from 3-60 mg were safe and well-tolerated in 36 healthy volunteers.17