
Due to its wide net efficiency in intercepting various addictions, ibogaine has been used by shamans in West Africa for many centuries.1,2 It is becoming a larger player in the pool of sexy psychedelics to research, due to this rare efficacy and broad use. Isolating the ibogaine alkaloid, found most commonly in the Tabernanthe iboga plant, faces ethical and environmental challenges.1,3 Despite its intriguing qualities, this makes it difficult to engage as a viable compound to assist in the epic, wide-range battle of addiction.

Figure 1: Click to enlarge. The molecular structure of ibogaine and various Iboga-type alkaloid molecules. Public domain images compiled from Wikipedia.
There are two ways to isolate an alkaloid like ibogaine: removing the existing molecule from its plant of origin or building the molecule from look-alike alkaloids. Dr. David Olson’s lab at the University of California Davis has been looking into the latter, using Iboga-type alkaloids, hoping to circumvent these extraction challenges faced by the Iboga plant.4
Iboga-type alkaloids are structurally and/or neurochemically similar to ibogaine. Hundreds of compounds like voacangine and coronaridine have been identified (Figures 1 and 2), yet limitations on synthesis efficiency still remain. Ibogaine has traditionally been extracted from plants found in western parts of Africa. But in the past 10 years, more plants in Mexico, China, and Malaysia that contain Iboga-type alkaloids have been identified.4 The goal is to use other plants, or series of plants, to mine for these alkaloids.

Figure 2: Click to enlarge. The chemical yields of different compounds found in various plant sources show the potential for ibogaine and Iboga-type alkaloid isolation.4
Why Are Researchers Willing to go to Such Great Lengths to Isolate Ibogaine?
Ibogaine is unique in its ability to physiologically reduce withdrawal and psychologically initiate neuroplastic changes on broad addictions.5,6 The known neurobiological circuitry of ibogaine is confusing, to say the least. Various research has shown its ability to reverse addiction to opiates, stimulants, alcohol, and nicotine.5-8 Ibogaine and noribogaine, bind promiscuously with weak strength to a number of receptors across the brain – serotonin, opioid, acetylcholine, sigma, and NMDA receptors, affecting both neurotransmitter release and transport.4
The impacts of ibogaine on brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) are lead players in contributing to broad structural brain changes. Ibogaine regulates BDNF and GDNF expression in brain regions involved in the pathophysiology of addiction and has led researchers to hypothesize their involvement with some of the long-lasting effects observed.9,10
Dr. Olson’s lab has been looking into psychoplastogens, small molecules that can quickly regulate larger molecules like BDNF and GDNF expression, upon a single administration, with sustained effects. Ibogaine has been shown to be a promising psychoplastogen, efficient at rewiring characteristically stubborn neural circuits, in a circuit-specific manner.9,11 New Iboga-type alkaloids could provide relief to the environmental complications of harvesting the Iboga plant, as well as help characterize unknown biosynthetic pathways.
What Makes a Good Ibogalog?
Things get more complicated as the cardiotoxicity of ibogaine is well known to researchers, landing it in the Schedule 1 category of narcotics.12 Iboga-type alkaloids (ibogalogs) are novel variants on the ibogaine molecule, which may produce similar effects on substance use disorder but lack the health risks to the heart. In addition to easier extraction, the goal is for researchers to identify a group of ibogaine analogs, that produce less of the undesired side effects.4
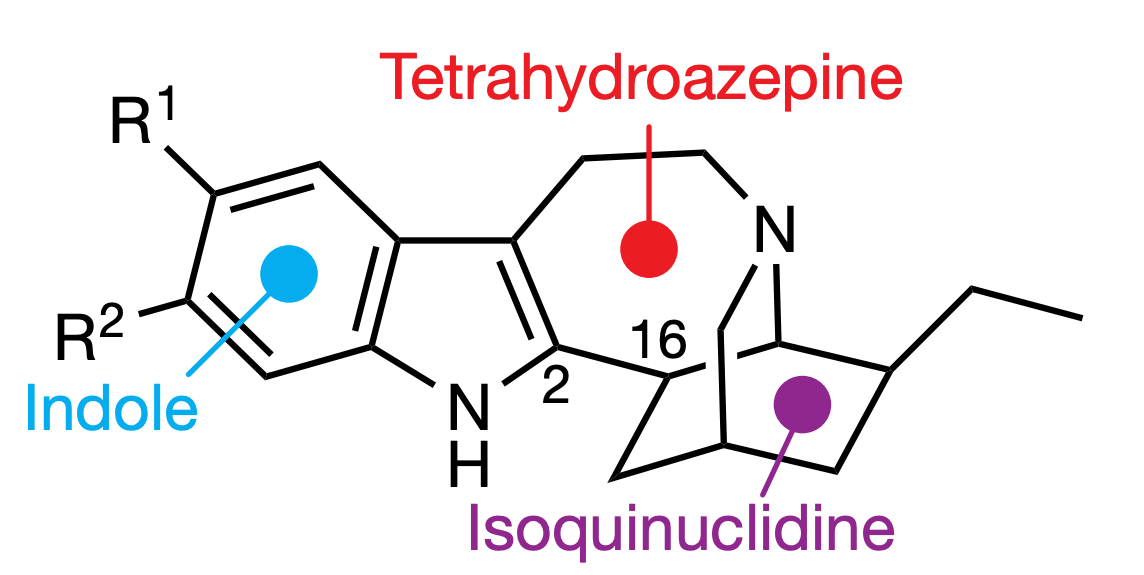
Figure 3: Click to enlarge. Carbon ring systems that are the molecular focus for function-driven ibogaine-like alkaloids, or ibogologs.13
With ibogaine’s mechanism being poorly defined, the understanding of what makes a good molecular structure is missing. Because ibogaine-like effects centered around some difficult to synthesize carbon ring systems (isoquinuclidine ring system, tetrahydroazeepine, and indole rings), it is difficult to allow the function to drive the synthesis of new molecules (Figure 3).
Dr. Olson’s team has started to develop and sift through some of the potential ibogalogs, which vary in their binding affinity profile, thought to be a major contributor to mimicking ibogaine like effects.4 Few of these have been found and tested, as it is hard to know what to look for in a dark room. The compounds 18-MC and 18-MAC (Figure 4) are among some of the more researched ibogalogs, although Olsen’s team poses additional promising structures for research.1
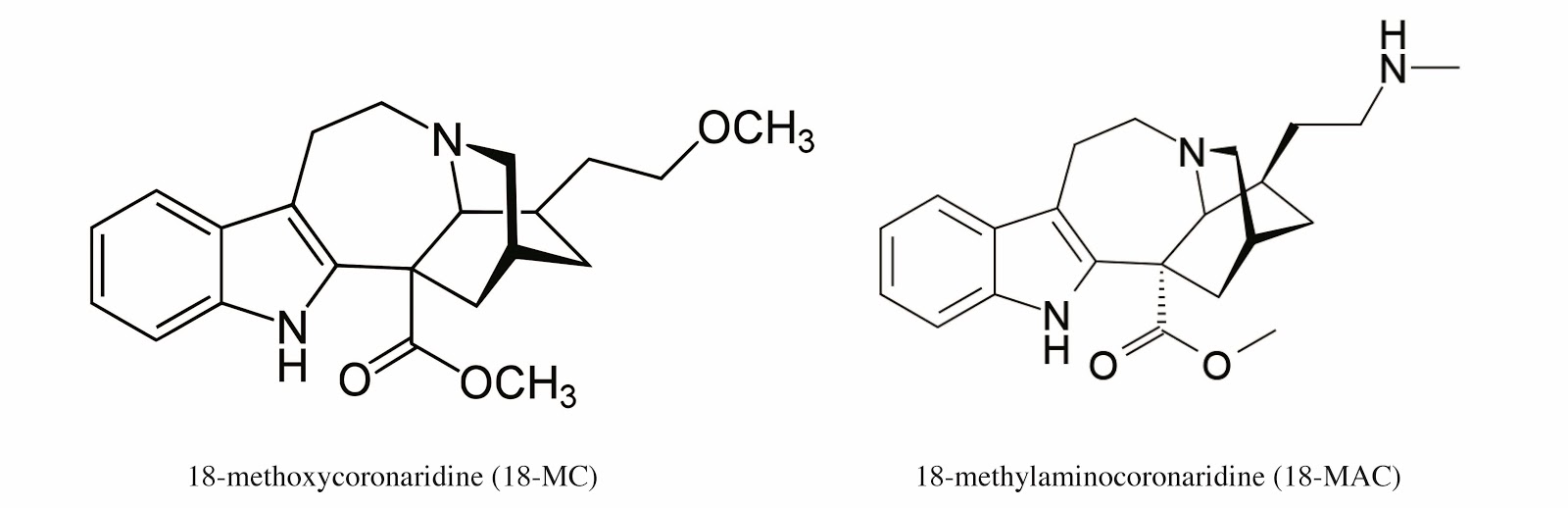
Figure 4: Click to enlarge. The chemical structure of 18-MC and 18-MAC. Public domain images compiled from Wikipedia.
Summary
The unknown mechanistic nature of ibogaine’s effect on the brain, combined with the difficult structural synthesis, impairs the ability to functionally or biomimetically explore Iboga alkaloids at a rapid rate. Despite this difficulty, studying these unique compounds’ ability to work globally on addiction pathways, rather than locally on substance-specific ones, is what drives this research.
Exploring various Iboga alkaloids promotes the beginning of a wave of research that identifies substance use disorder treatment as less substance-specific. This allows Western medicine to search for new and more effective drugs while respecting the ethical boundaries of the source.
Well written, concise, understandable.