
Psychedelic compounds have been investigated for a variety of anxiety and depression-related disorders but some come with unwanted side effects such as hallucinations.1 Recently, a research team from Olson Lab led by Lindsay Cameron synthesized a novel non-hallucinogenic analog of ibogaine that has therapeutic properties.2
Study Design – Using Mice, Rats, and Zebrafish
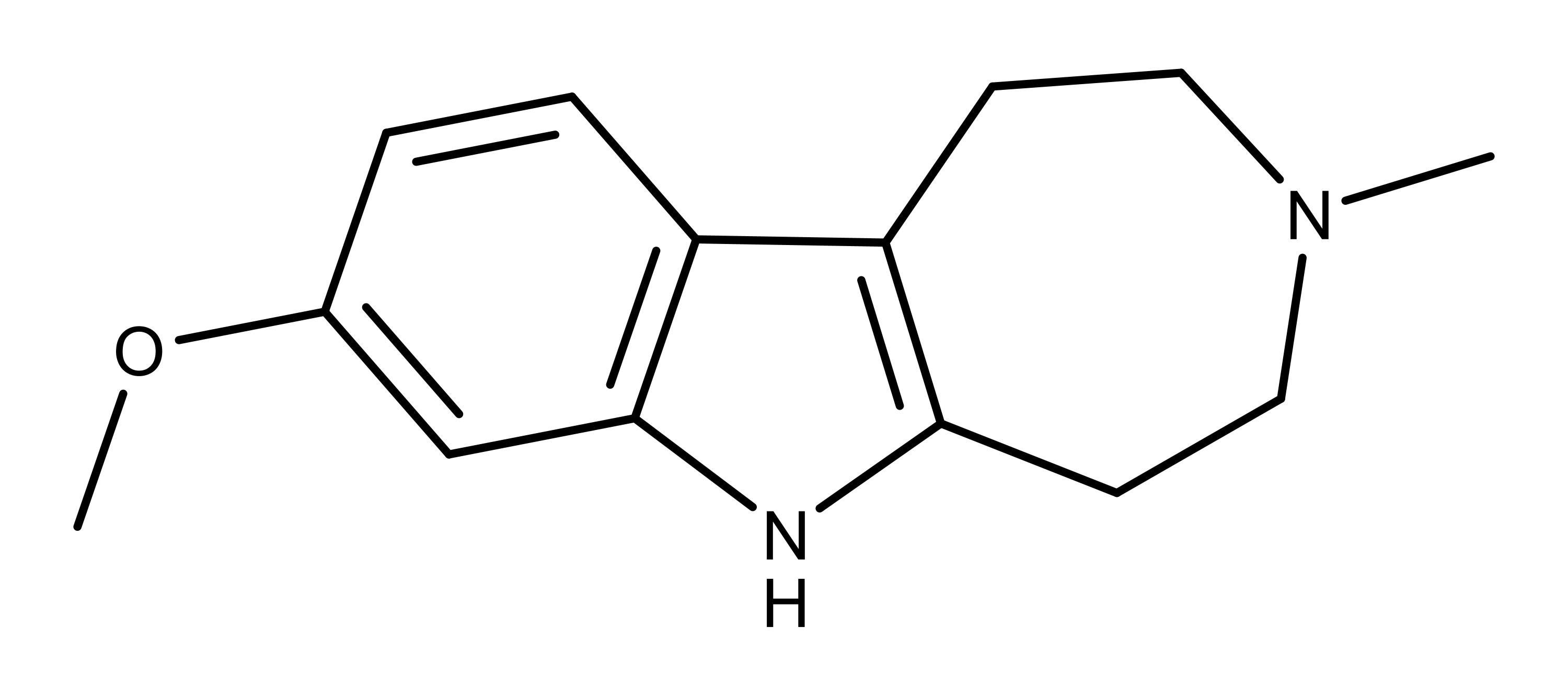
For creating an analog of ibogaine, the researchers focused on “function-oriented synthesis,” which identifies structural features of the compound that are important to its function. Deletion of specific structural elements provides the core of the compound. Using this approach, Cameron et al. created analogs of ibogaine and assessed them for psychoplastogenic properties. They found one analog with a simplified structure, ibogainalog, showing promising plastic effects. Using this ibogainalog backbone and structure-activity relationships, they synthesized a compound that they named tabernanthalog.
To test the toxic effects of tabernanthalog, the authors used a zebrafish model. Zebrafish are a commonly used animal model to test the toxic effects of compounds.3 These animals possess a similar potassium channel to humans that regulates cardiac activity. The researchers used a whole-cell mode patch clamp for assessing heart rate and the inhibition of ion channels. This method allows experimenters to administer the drug directly to the cell and measure the channel activity.
For evaluating changes in heart rate, they exposed zebrafish larvae to the drug and filmed regions of interest, followed by code calculation of peak time interval, heart rate, and arrhythmias (zebrafish larvae are nearly transparent, making it possible to quantify changes in heart rate). The researchers also tested for seizures through the administration of compounds to fluorescent expressing larvae. Using video software, a change in fluorescent intensity was calculated. For determining larval toxicity, the fish were exposed to compounds for 6 hours through 5 days post-fertilization. Following exposure, the larvae were examined for mortality and abnormal formations.
Tabernanthalog was then tested alongside ibogainalog, ibogaine, and DOI (positive control) for neuroplasticity effects. Cells from rat embryos were cultured and stained to allow quantification of dendritic spines. They also used live animals to assess spine dynamics, where they were able to visualize the same dendritic spines and quantify the formation/elimination of the spines 24 hours post-administration.
Following confirmation that tabernanthalog has psychoplastogen effects via the cell culturing tests, the researchers utilized an unpredictable mild stress model where mice go through a week of being exposed to different stressful stimuli like light or noise. Following this, mice were given tabernanthalog or a placebo and tested in the forced swim test where the immobility time of the mice was measured.
To evaluate the effects of the compounds on binge drinking, Cameron et al. used an intermittent ethanol paradigm where the mice received alcohol every other day. Mice chose between water or alcohol and they measured how much was drunk from each bottle. Following drug administration, the paradigm was changed and ethanol was left on for 48 hours at a time. Ethanol was measured at four, 24, and 48 hours. Assessing whether tabernanthalog would alter preference to sucrose or water was studied using a two-bottle choice paradigm. Here, the mice could drink from either bottle. Sucrose preference was calculated as the amount of sucrose solution consumed minus the amount of water consumed, divided by the total amount of liquid consumed
They also tested how tabernanthalog administration affects heroin self-administration using a choice-model where rats could self-administer heroin or control using levers. This was followed by the extinction of heroin self-administration where levers did not produce the drug and a cue-light reinstatement to see if rats would reestablish lever pressing.
Major Findings Show Reduced Side Effects
The study reports that tabernanthalog did not induce irregularity in heart function or seizures and produced fewer morphological abnormalities when compared to ibogaine. Also, lower concentrations of tabernanthalog were found to be similar to the control in morphological experiments.
Tabernanthalog promoted dendritic growth in rat embryonic cortical neurons and increased density of dendritic spines, comparable to ibogaine. In the assessment of spine dynamics, tabernanthalog treatment increased overall spine formation without an effect on spine elimination. These results were observed in the cortex, consistent with reports of ketamine and other psychedelics’ effects.4 The novel compound also increased the mobility of previously stressed mice in the forced swim test.
In the drinking experiments, tabernanthalog reduced binge drinking during the first four hours of the session, and alcohol consumption was lower 48 hours following tabernanthalog without altering sucrose preference. In opioid self-administration, tabernanthalog acutely reduced heroin-seeking behavior but also reduced sucrose preference. In the cue-induced relapse experiment, tabernanthalog treatment reduced relapses for a period lasting up to 14 days.
Tabernanthalog vs. Ibogaine – A Critical Comparison
Anecdotal reports have suggested ibogaine reduces drug withdrawal symptoms and is effective in preventing relapse. However, it has adverse side effects, such as hallucinations lasting greater than 24 hours.5 Cameron et al. observed that tabernanthalog did not produce effects in the head twitch response assay that tests for hallucinogenic activity.
In addition, several deaths have been reported after ibogaine use due to its severe cardiotoxic effects.5 The data from this study showed that tabernanthalog is less cardiotoxic than ibogaine due to its lack of effects on the channels that regulate heart activity.
Study Limitations
Although the work by Cameron et al. provides an in-depth framework for synthesizing functional, non-hallucinogenic analogs of psychedelics, the basic pharmacology of tabernanthalog still needs to be investigated. The authors did include some pharmacological profiling data in their extended data, but further tests should explore the time course of the compound and dose-response curves to see the highest effective and toxic doses. Ibogaine has complex pharmacology and is rapidly metabolized, so these analogs may share that complexity.
Moreover, further research is necessary to determine downstream signaling and if structural plasticity is causal to behavioral changes. While this paper used multiple animal models, replication of results and further assessment of behavioral effects is necessary.
Summary and Conclusions
This paper utilizes a basic principle of science: structure determines function.6 Using structure-activity relationships and behavioral tests in multiple animal models, Cameron et al. determined the structural core necessary for therapeutic effects of ibogaine while altering the structure to decrease adverse side effects. This novel compound, tabernanthalog, has been shown to have effectiveness as a psychoplastogen without hallucinations or cardiotoxicity.
While there is more work to be done to fully characterize this compound, psychoplastogenic medicines could produce sustained therapeutic effects by targeting underlying changes in circuitry, not just addressing symptoms of the disease. In addition, these findings beg the question: are mystical experiences necessary for positive therapeutic outcomes?7

Great summary of a very interesting topic. Thank you.