
2C-B is a drug that belongs to the phenethylamine family. Figure 1 shows its chemical structure. Alexander Shulgin first synthesized 2C-B in 1974. This synthetic drug has been receiving a lot of attention recently due to its increasing popularity in the club scene along with ketamine, MDMA, and cocaine.
Drugs in the phenethylamine family are stimulants of the central nervous system. Because of this, it makes sense that at first glance compounds like 2C-B are not considered psychedelic drugs like tryptamine compounds (e.g., psilocin, bufotenin). However, further examination of 2C-B shows it is indeed psychedelic in terms of its effects and receptor binding.
The subjective effects of 2C-B are similar to other psychostimulants like MDMA and amphetamine and psychedelics such as ayahuasca, salvinorin A, and Salvia divinorum.1 The effects include euphoria, mood changes more than perceptual changes, and a psychedelic experience with an altered state of consciousness.
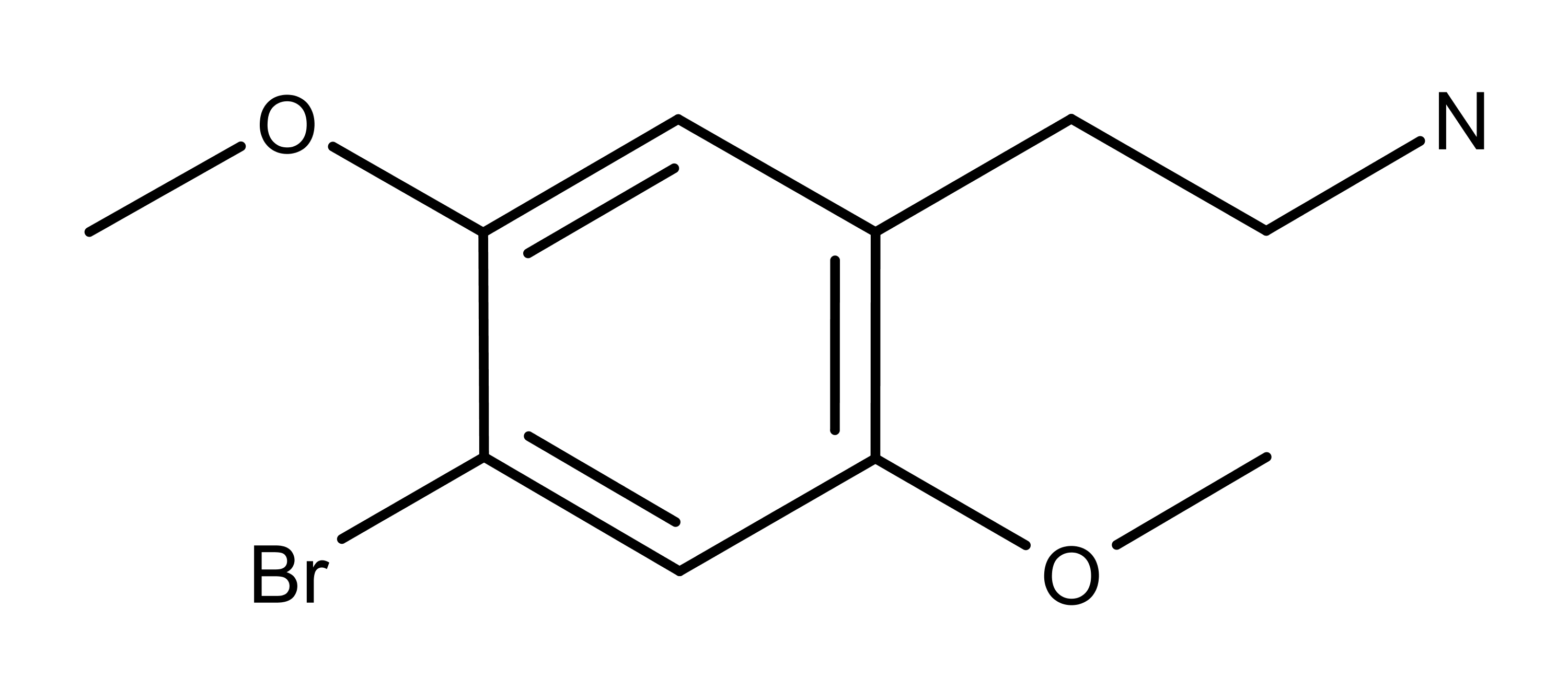
Figure 1: The chemical structure of 2C-B. Its chemical name is 2-(4-bromo-2,5-dimethoxyphenyl)ethanamine.
Scientists classify a drug as psychedelic if its effects are caused by agonist or partial agonist binding to serotonin 5-HT2A receptors.1,2 No fMRI studies have been done to confirm 2C-B binding to 5-HT2A receptors in the human brain correlates with subjective effects. However, testing using 5-HT2A in in vitro receptor affinity studies confirms this theory. In 2010, the Psychoactive Drug Screening Program (PDSP) at the National Institute of Mental Health assayed 25 drugs including 2C-B at 51 receptors.3 The results showed 2C-B is an agonist of 5-HT2A.
In addition, the affinity of 2C-B for 5-HT2A has been shown in oocytes from the frog Xenopus laevis (African clawed frog)4 and Chinese hamster ovary K1 cells5 expressing the human 5-HT2A receptor. These studies categorized 2C-B as a partial agonist of 5-HT2A. There is also experimental evidence indicating 2C-B is a partial agonist of 5-HT2B and 5-HT2C.6,7 However, it is unknown how the agonist activity at these two receptors influences the psychedelic effects of 2C-B.
Although it is not a tryptamine, 2C-B is a psychedelic drug. The evidence for this comes from scientific studies showing its affinity for the 5-HT2A receptor. Also, experience reports from users of 2C-B have documented its effects which include a psychedelic experience. Further research into 2C-B would provide insight into the possible therapeutic potential of this compound.

Who ever wrote this has obviously never tried 2CB. It is nothing like salvia, or amphetamine. Even the comparison to ayahuasca is a stretch of the imagination. It is somwhere between MDMA and psilocybin, 4-6 hours of beautiful visuals and euphoria, however the visuals are not like psilocybin, more complex geometry and more sparkle. The euphoria is not lovey dovey like MDMA, as it has an erotic edge. It’s most similar to mescaline both in structure and spiritual profoundness. I also think as a partial agonist is has antagonistic activity as well at the 5HT- receptors. 2CB is most definitely… Read more »
Hi Dave – Thanks for your comments. My article says that 2C-B has psychedelic effects and is an agonist at the 5-HT2A receptor. Therefore, I’m not sure why you thought otherwise (and accused me of spreading misinformation) after reading it. The Papaseit et al. study is the source of the information about the subjective effects of 2C-B being similar to salvia, ayahuasca, amphetamine, etc. Perhaps you should address your comments about the effects to the authors.
I took some 2cb a few hours ago. Tried it before a number of times. Reminds me when acid is about to peak but never quite gets there . Visuals are definitely LSD like energy is up there. In my honest opinion take this on a sunny day or at sun set for the best visuals. The headspace definitely Reminds me of LSD it has that familiar LSD sheen to everything you look at.
As with everything else enjoy responsibly and research your doses before you commit yourself to the experience ain’t going back once you consume it.
What do you make of Villalobos et al. (2004), and previously Acuna-Castillo et al. (2002), reporting negligible intrinsic activity or antagonist activity of 2C-B at 5HT2AR heterologously expressed in Xenopus laevis oocytes? This seems to conflict with the efficacy reported by Ray (2010), who measured calcium release – I’m having trouble identifying the exact system Ray (2010) used because there are dead-links to websites describing detailed methodology appearing in two papers, but I presume it is also a heterologous expression system (HEK293 maybe?). I’ve never found anybody examining the mouse head-twitch response to 2C-B either. It seems like a bit… Read more »