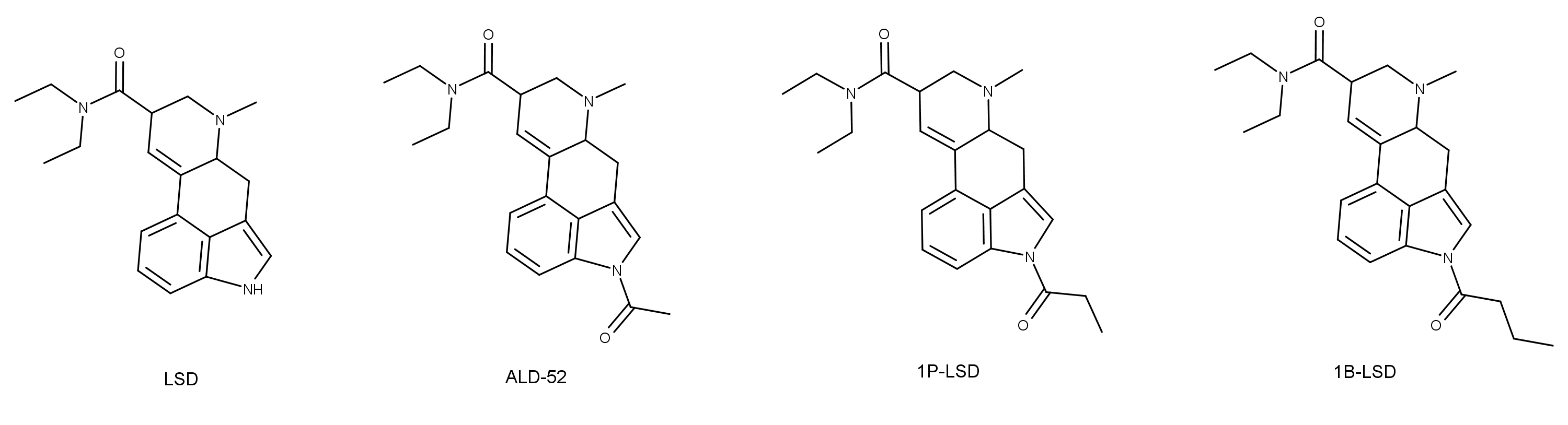
In a November 2019 study published in Neuropharmacology, researchers tested whether the LSD (lysergic acid diethylamide) derivatives ALD-52 (1-acetyl-LSD), 1P-LSD (1-propanoyl-LSD), and 1B-LSD (1-butanoyl-LSD) are active compounds or prodrugs of LSD.1 The scope of the study included learning about the pharmacological effects and mechanism of action of these 1-acyl-substituted LSD derivatives.
Study Design
The study used a three-pronged approach for testing the LSD derivatives: competitive binding studies and calcium mobilization to examine the interaction with serotonin receptors, head twitch response (HTR) studies in mice to assess in vivo activation of serotonin 5-HT2A receptor, and LC/MS (liquid chromatography/ion trap mass spectrometry) to quantify the amount of LSD in the blood of rats treated with two of the three derivatives.
Serotonin Receptor Interaction and Activation
The binding affinity data indicated that the 1-acyl substitution on the LSD derivatives reduced their affinity for 5-HT1A compared to LSD (9.5 nM). The magnitude of the effect depended on the length of the acyl group (note that the higher the Ki, the less affinity the compound has for the receptor):
- 1B-LSD: Ki = 345 nM = 36-fold lower affinity than LSD.
- 1P-LSD: Ki = 637 nM = 67-fold lower affinity than LSD.
- ALD-52: Ki = 1,054 nM = 111-fold lower affinity than LSD.
For 5-HT2A, an acetyl or propanoyl group on LSD’s indole nitrogen reduced receptor affinity by more than 10-fold compared to LSD (14.7 nM). Substitution with a butanoyl group reduced affinity around 5-fold:
- ALD-52: Ki = 174 nM
- 1P-LSD: Ki = 196 nM
- 1B-LSD: Ki = 87.7 nM
Interestingly, the 1-acetyl substitutions increased the affinity of the derivatives at 5-HT2C by about 2-4-fold compared to LSD (45.3 nM):
- ALD-52: Ki = 10.2 nM
- 1P-LSD: Ki = 13.0 nM
- 1B-LSD: Ki = 20.8 nM
After determining the receptor binding affinity for the three LSD derivatives, tests using Gq-mediated Ca2+ flux in HEK cells (calcium mobilization) examined whether the derivatives activated the serotonin receptors.
The data showed that ALD-52, 1P-LSD, and 1B-LSD were very weak partial agonists at the human 5-HT2A compared to LSD. Interestingly, despite showing comparatively high affinity for 5-HT2B and 5-HT2C, the compounds showed no agonist activity at those receptors. This behavior is in contrast to LSD, which is an agonist at recombinant human 5-HT2B and 5-HT2C receptors.
The study also assessed the binding affinity of 1B-LSD for 24 other monoamine receptors. Overall, the data indicated that 1-butanoyl substitution had a detrimental effect on binding to most of the receptors, compared to previously reported data for LSD. The binding affinity at these receptors was 10-100-fold lower than for LSD. The two main exceptions were 1B-LSD binding at the 5-HT2C receptor, as discussed earlier and 5-HT2B.
HTR Testing
Although the data indicated the 1-acyl substitution on the derivatives reduced their affinity and efficacy at 5-HT2A, ALD-52, 1P-LSD, and 1B-LSD still induced head twitches in mice and showed a relatively high potency compared to other hallucinogens. The authors noted that “…the rank order of potency of the four lysergamides is inversely proportional to the length of the substituent present on the indole nitrogen.” ALD-52 had about half the potency of LSD and 1P-LSD about one-third. The potency of 1B-LSD was only 14% of LSD.
LC/MS Analysis
The authors reported that the LC/MS analysis indicated, “High levels of LSD were detected in the plasma of rats after subcutaneous administration of ALD-52 and 1P-LSD, demonstrating these compounds are rapidly and efficiently deacylated in vivo.” The authors noted that ALD-52 and 1P-LSD appeared to undergo deacetylation at roughly the same rate. This theory was evidenced by the rats have almost identical plasma levels of LSD after being treated with either compound.
In addition to LSD, the analysis also detected ALD-52 and 1P-LSD in the plasma along with several of their metabolites. The data correlated with previously published results for rats and humans.
The Importance of Identifying and Understanding Prodrugs
Prodrugs expand drug formulation options by having different solubilities and other physical properties. A prodrug can take the place of a drug that doesn’t have adequate pharmacokinetic or biopharmaceutical activity in the body. They can also provide solutions for compounds that are toxic or have undesirable side effects. Prodrugs may also present an array of options when it comes to harnessing the entourage effect for developing targeted formulations.
The principal finding of this study is that ALD-52, 1P-LSD, and 1B-LSD are prodrugs of LSD. Additional aspects of this work indicate that 1-acyl substituted LSD derivatives are potent hallucinogens despite having less efficacy at the 5-HT2A receptor. Scientists will apply these findings and design new experiments that will answer (and, as is the nature of science, create) additional questions about how psychedelic drugs work.