When it comes to the psychedelic experience, the serotonin 5-HT2A receptor (5-HT2AR) receives most of the attention, and rightly so. A 2019 study showed that the psychedelic effects of psilocybin were correlated with the levels of psilocin in the blood of volunteers and psilocin occupancy of 5-HT2AR in their brains.1 But what about the other 13 serotonin receptors? Do any of them contribute to the effects that psychedelics have on the user? How? Do these receptors modulate the effects of other serotonin receptors or other receptor families? Some clues to answering these questions come from receptor binding affinity data.
Subtle Differences in Compound Chemistry = Significant Effects
The neurotransmitter serotonin and psychedelics such as psilocybin, psilocin, LSD (lysergic acid diethylamide), DMT (dimethyltryptamine), and 5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine) have similarities and differences in their chemical structures (Figure 1). The differences in the structures may appear subtle, but small changes in the chemistry of compounds affect how they are metabolized in the body, and ultimately, their effects on the user. On top of this, these psychedelic compounds have an affinity for several serotonin receptors (aka 5-HT receptors), some more than others. So, understanding serotonin receptors is critical for understanding how psychedelic drugs work.
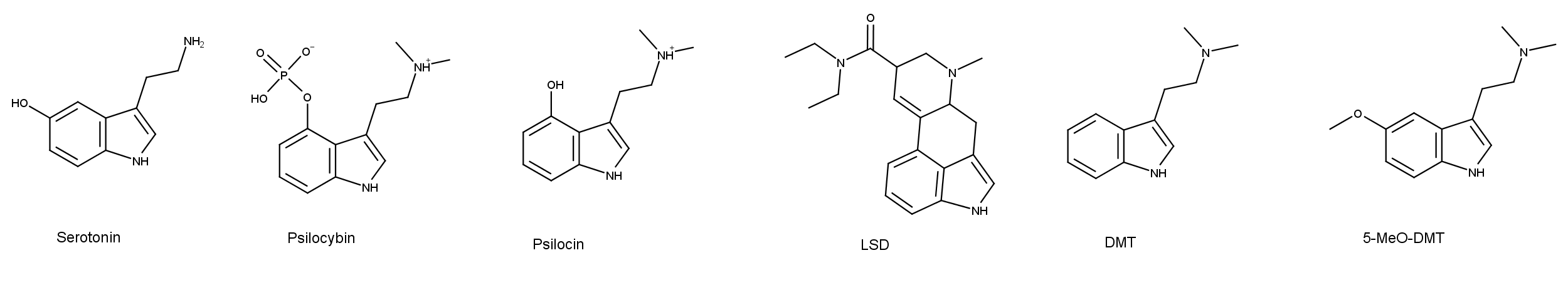
Figure 1: The chemical structures of serotonin and five psychedelic compounds. Note the indoleamine skeleton in the serotonin molecule is also present in the other compounds (click to enlarge).
G Protein-Coupled Receptors and Allosteric Modulation
Two concepts are important for interpreting the data in Table 1; GPCRs and allosteric modulation. Serotonin receptors belong to the G protein-coupled receptor (GPCR) family.2 GPCRs are responsible for mediating most of the cellular responses to hormones and neurotransmitters. They are also involved in vision, smell, and taste. One interesting aspect of GPCRs is that they are subject to a phenomenon called allosteric modulation.3 Allosteric modulators are small molecules that do not bind to the primary binding site (aka the orthosteric site) on GPCRs, but rather an alternate binding site (the allosteric site). When this binding occurs, the receptor changes conformation (i.e., changes shape). This alters how the GPCR interacts with a different molecule (also called a ligand) at the orthosteric site.
Receptor Binding Affinity – Where Less is More
Scientists test how well drugs and chemicals bind to receptors by measuring their binding affinity, designated by the symbol Ki. Binding affinity is one kind of dissociation constant. This means that the higher the number, the more likely the substance is to separate from the receptor. Conversely, low binding affinity values indicate the compound binds more strongly and is less likely to dissociate from the receptor. These binding affinities are often measured in nanomoles (nM). Table 1 below shows the Ki values for serotonin and five psychedelic compounds.
Table 1: Ki values for various psychedelic compounds and serotonin receptors. An asterisk denotes non-human data. The citation for each data point is in parentheses. Values >10,000 are considered negative results (no binding affinity).
| Receptor | Psilocybin Ki (nM) | Psilocin Ki (nM) | LSD Ki (nM) | DMT Ki (nM) | 5-MeO-DMT Ki (nM) |
|---|---|---|---|---|---|
| 5-HT1A | >10,000 (6) | 567.4 (6) | 1.1 (7) | 119.5 (12) | n/a |
| 5-HT1B | >10,000 (16) | 219.6 (6) | 3.9* (8) | 2,200* (13) | 351* (11) |
| 5-HT1C | >10,000* (16) | n/a | 5.5* (7) | n/a | n/a |
| 5-HT1D | 2119 (16) | 36.4 (6) | 14.0 (8) | 270 (8) | n/a |
| 5-HT1E | 194.8 (16) | 52.2 (6) | 93.0* (2) | n/a | n/a |
| 5-HT1F | n/a | n/a | n/a | n/a | n/a |
| 5-HT2A | >10,000 (16) | 107.2 (6) | 3.5* (14) | 230 (12) | 207 (16) |
| 5-HT2B | 98.7 (6) | 4.6 (6) | 3.7 (9) | 550* (14) | 1,300 (14) |
| 5-HT2C | >10,000* (6) | 97.3* (6) | 5.5* (7) | n/a | 100* (17) |
| 5-HT3 | >10,000 (6) | >10,000 (6) | >10,000* (10) | n/a | n/a |
| 5-HT4 | n/a | n/a | n/a | n/a | n/a |
| 5-HT5A | n/a | n/a | 9.0* (7) | n/a | n/a |
| 5-HT5B | n/a | n/a | 3.2 (11) | n/a | n/a |
| 5-HT6 | 413.5 (6) | 57.0 (6) | 6.9* (7) | 68.0 (15) | n/a |
| 5-HT7 | 597.9 (6) | 3.5 (6) | 6.6* (7) | n/a | n/a |
Some Insights from the Receptor Binding Data
The data shown in Table 1 has some limitations, including the use of different species and radioligands. However, some interesting generalizations appear from studying the data. For example, the binding affinity data for psilocybin and psilocin at 5-HT2AR shows how psilocybin is a prodrug of psilocin, the active metabolite responsible for the psychedelic effect (i.e., psilocin has a significantly lower Ki than psilocybin).
Looking at the data for just psilocybin in Table 1 reveals some interesting tidbits of information. Notice that psilocybin has some modest binding activity at 5-HT1D, 5-HT1E, 5-HT2B, 5-HT6, and 5-HT7. This indicates that the natural compound psilocybin, which is virtually inactive at 5-HT2A, does not require metabolic dephosphorylation to have binding activity at other serotonin receptors.
Psilocin, LSD, DMT, and 5-MeO-DMT have different binding affinities at all the serotonin receptors at which they were tested except 5-HT3 (discussed later). These data present a myriad of questions about the contribution of these receptors to the entourage effect, and how they contribute to the overall psychedelic effect.
Psychedelic compounds such as those in Table 1 may also act as allosteric modulators at serotonin receptors. So, when a person ingests psychedelic mushrooms (aka magic mushrooms), for example, they are ingesting a cocktail of compounds. The data in Table 1 indicate that the psilocybin and psilocin could be binding to several different serotonin receptors. Compounding those effects is that the receptors could also be influenced by allosteric modulation by other chemicals in the cocktail.
Another interesting observation from the data in Table 1 is the binding data for 5-HT2A compared to 5-HT2C. In a 2004 paper in Pharmacological Reviews, Dr. David Nichols observed that all psychedelics (to this point) are known agonists at 5-HT2A and 5-HT2C.4 He says,
…higher doses of particular psychedelics may lead to activation of the 5-HT2C receptor, which often functionally opposes the effects of 5-HT2A receptor activation.
These are fascinating ideas that may help in understanding the entourage effect in magic mushroom compounds.
5-HT3R is different than the other serotonin receptors, as evidenced by its lack of binding affinity shown in Table 1. As opposed to other serotonin receptors that are GPCRs, 5-HT3R is a ligand-gated ion channel.5 This means that when it is activated by agonist binding, channels open allowing ions (such as sodium, potassium, and calcium) to flow in and out of the cell. With neuron cells, this ion flow causes an excitatory response. To date, research shows 5-HT3 is found mostly in the gut, and it is used as a drug target for treating conditions such as nausea and vomiting and irritable bowel syndrome.
The data for LSD at the serotonin receptors is fascinating. Unlike magic mushrooms or toad secretions, LSD is a synthetic compound made in the lab. Notice that LSD has a lower binding affinity for serotonin receptors than most of the other compounds shown in Table 1, meaning it binds more strongly.
Finally, the data in Table 1 reveals opportunities for research studying some of these psychedelic compounds at 5-HT1F, 5-HT4, 5-HT5A, and 5-HT5B. Even if binding affinity tests indicate these compounds have no activity at these receptors, that is still valuable information for integrating into the understanding of the entourage effect in these natural compounds. Also, these compounds could act as allosteric modulators in influencing the overall psychedelic effect.
More Research is Needed on Serotonin Receptors and Psychedelics
Much remains to be learned about serotonin receptors and how they function under the influence of psychedelics. Naturally occurring organisms like magic mushrooms and psychedelic toad secretions contain a variety of different compounds that may be important in the entourage effect. Even compounds in these organisms that are not active at orthosteric receptor sites may be crucial to the overall effect because of allosteric modulation. This is a wide-open area of study for the curious researcher with important applications for making formulations with measured amounts of selected compounds.

If these drugs had been allowed to be researched here in USA, instead of offshore labs in other countries, we would be much farther ahead in our understanding the of roles these 15 variations of the 5Ht serotonin receptors play in these remarkable drugs, the UN treaties against all research did’n’t help either. All the laws did is shut down the research labs, and the black market set up shop. These drugs don’t belong manufactured in underground labs, they are major medicines and should never fallen into the legal system. In Oregon we just decriminalized possession of limited amounts of… Read more »
It seems to have an error with the number for 5ht-1a and psilocin, your other article (in this Web site*) shows a value of 49, instead of 567.4 (6).
*”Binding of Psilocin and Psilocybin to Serotonin Receptors”, February 26, 2019
Very, very interesting stuff btw ! Thank you for this. I wonder if a study can be made to link subjective reported effects with binding affinity of differents substances. Feeding a machine learning program with trip repports and receptor binding association might lead to more clues about their roles.
I could be wrong but I believe there is a minor mistake in the sentance: “Notice that LSD has a lower binding affinity for serotonin receptors than most of the other compounds shown in Table 1, meaning it binds more strongly.”
If I’m not mistaken a lower binding affinity would mean LSD binds less strongly, I think you meant that it has a lower Ki value, meaning it binds more strongly.