Naturally occurring compounds and their potential recreational, medical, and therapeutic uses continue to fascinate and pique the curiosity of scientists, psychonauts, and everyday people. On the scientific side of things, researchers are continuing to discover new compounds in psilocybin (magic) mushrooms, cannabis, and other natural sources. These discoveries answer questions but also pose more and add to the complexity of understanding why nature is the way it is.
A recent discovery comes from October of last year, when a research team led by Yu-Lin Chen of Shenyang Pharmaceutical University in Benxi, China, published a paper in Molecules describing two new indole alkaloid compounds they isolated from the venom of the Asiatic toad Bufo bufo gargarizans.1
The team extracted compounds from the venom using multiple washes of distilled water. They employed several chromatographic techniques (e.g., HPLC, TLC) to separate out each compound and analyzed them using IR, NMR, and other spectral methods. They named the two new compounds they found bufotenidine B and bufocarboline A. Other known toad venom indole alkaloids they identified included N-carboxymethyl serotonin, bufoviridine, dehydrobufothionine, and bufobutarginine.
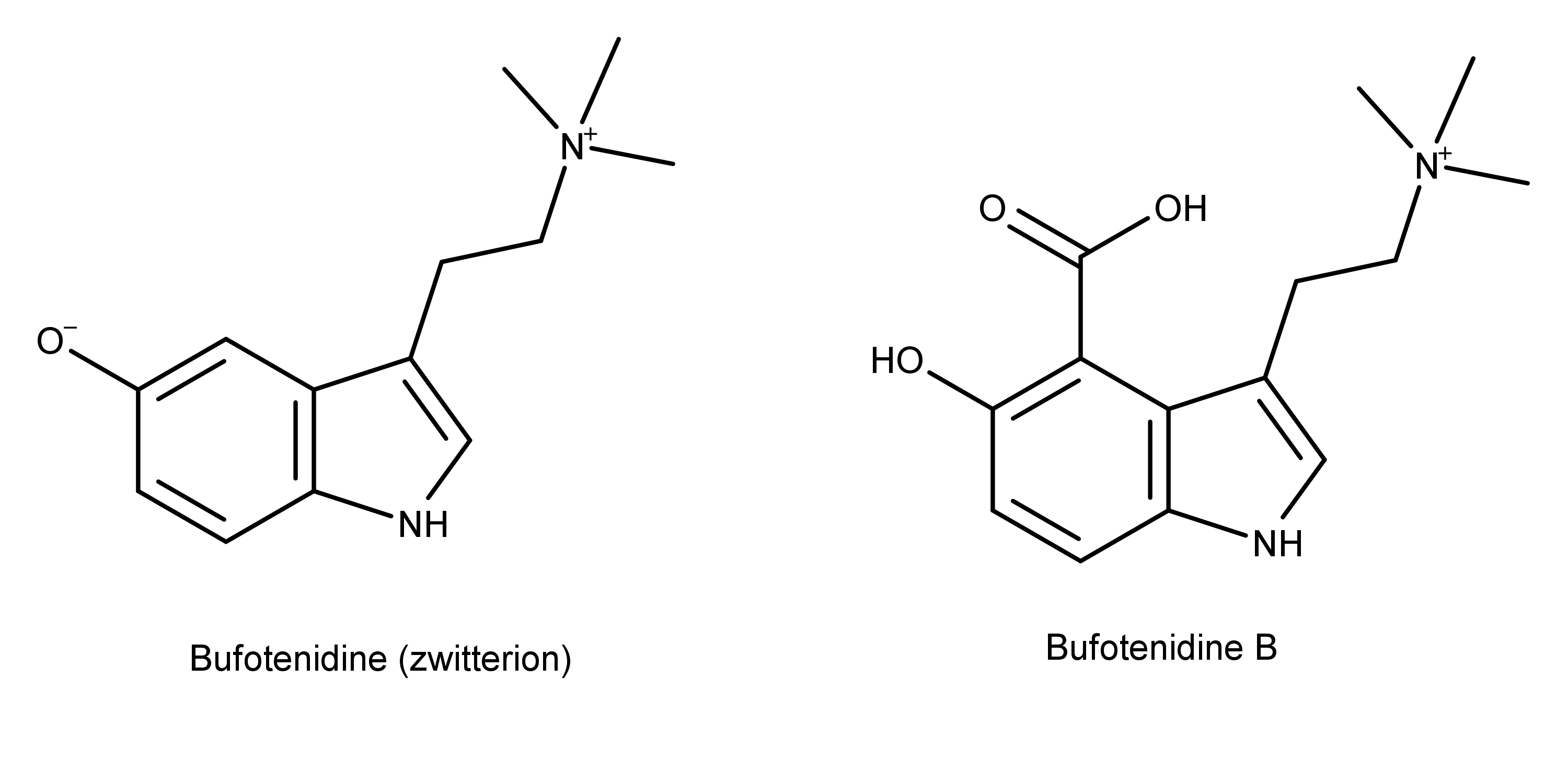
Bufotenidine B
This compound appeared as a yellow powder. Its chemical name is 5-hydroxy-3-(2-(trimethylammonio)ethyl) -1H-indole-4-carboxylate. The word bufotenidine will sound familiar to those who know about toad venom components. The image below shows the chemical structure of this more well-known compound alongside the newly discovered bufotenidine B.
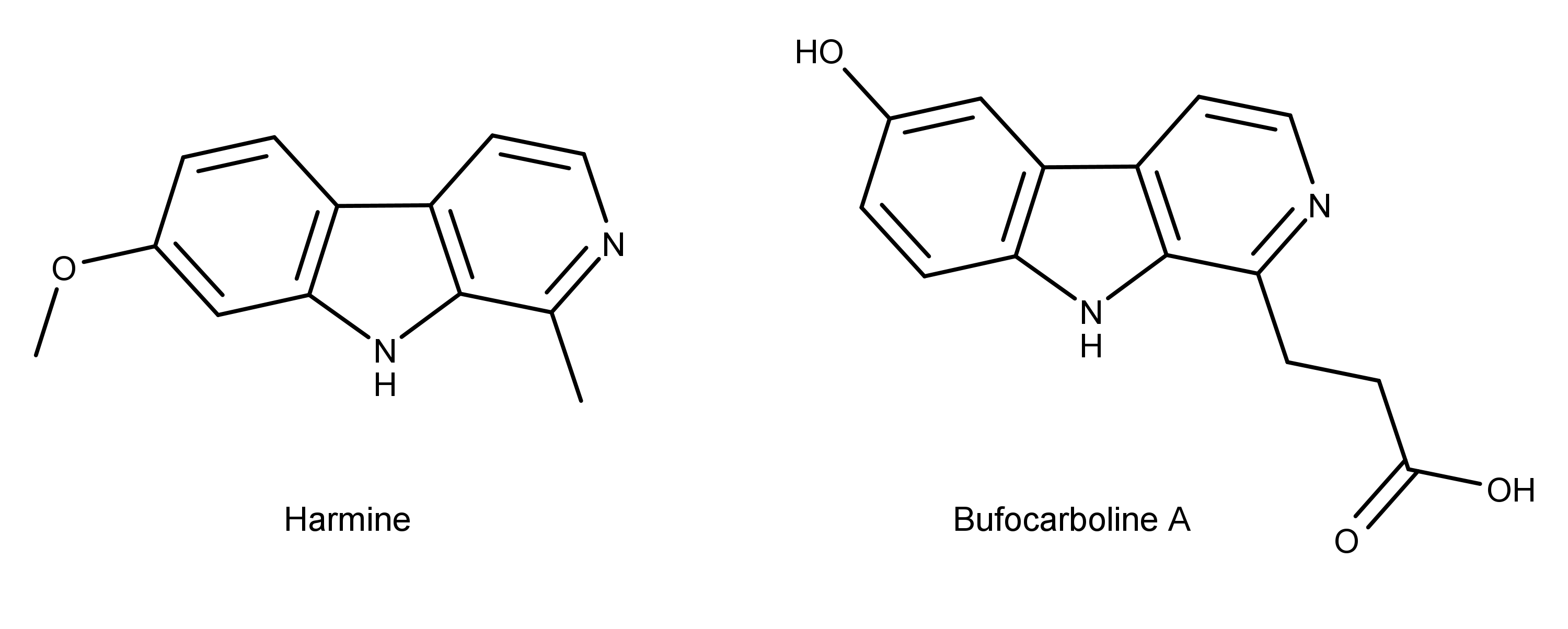
Bufocarboline A
Bufocarboline A turned out as a pale white powder. Its chemical name is 6-hydroxy-1-(20-carboxyethyl)-1,2,3,4-tetrahydro-ß-carboline. “-carboline” should also sound familiar to some. In the image below, notice the structural similarity between the newly discovered bufocarboline A and a ‘classic’ ß-carboline such as harmine, which is one of the main active compounds in ayahuasca (it’s a monoamine oxidase enzyme inhibitor, aka a MAOI).
Activity Against Cancer Cells
Chen et al. then tested the compounds for cytotoxic activity against human malignant melanoma cells A 375. They summarized the resulting data by saying, “Unfortunately, the experimental results disclosed that the IC50 values of them were greater than 100 µM, and no antitumor activity was observed.”
Summary and Discussion
This study presents fascinating new areas for psychedelic science exploration. For example, it would be interesting to find out if bufocarboline A is a MAOI like many other ß-carbolines.
Another important factor to consider in further research is the entourage effect. If the theory is true (scientists and R&D companies have opposing viewpoints right now), perhaps these compounds don’t have antitumor properties when tested individually. But what might happen when they are formulated into specific combinations? And do the ratios of the compounds impact the overall cytotoxic effects?
Another important consideration: Studies like this that use venom harvested directly from toads also bring awareness to what such practices may do to the toads and their environment. Psychedelic Science Review has previously reported on the efforts of people, including writer, scientific researcher, documentarian, and psychonaut Hamilton Morris, to protect toads and their habitats. Morris is a proponent of seeking synthetic routes for making these compounds so as not to disturb the toads yet carry on with further research. He even went as far as synthesizing the toad venom compound 5-MeO-DMT on an episode of his TV show Hamilton’s Pharmacopeia to demonstrate how easily it can be made.
These new indole alkaloid compounds isolated from toad venom put more pieces of the toad venom puzzle into place. They also give rise to new questions and paths for research, as is the nature of scientific investigation.