The 5-HT2A receptor is an essential component mediating the activity of classical psychedelics, those most-studied and clinically relevant, including LSD, DMT, psilocybin, and mescaline. This G-Protein Coupled serotonin receptor is essential for the changes in perception, cognition, and emotion stereotypically associated with psychedelic compounds.1 The last several years have seen a resurgence in interest regarding therapeutic applications of psychedelics, with a plethora of research having laid the groundwork for their modern study. Despite the promising results, there remain participants who do not respond to treatment and large proportions of subjects exhibiting no statistically significant benefits2; the reason for such variability in prognosis is currently unknown.
At the genetic level, Single Nucleotide Polymorphisms (SNPs) or random DNA sequence variations at a single position of the 5-HT2A receptor gene, HTR2A, may explain these individual variations in drug response. This research may be highly relevant as psychedelics are further studied, offering genetic insights into individual differences in safety and efficacy in humans. Notably, previous research has implicated SNPs in the HTR2A gene in varied responses to antipsychotic medications with mechanisms at this same receptor.3 Recent research published in ACS Chemical Neuroscience suggests that nonsynonymous SNPs (polymorphisms that alter the genetic code and result in the change of a single amino acid in the sequence) of the HTR2A gene may alter both the structure and function of the receptor, causing changes in pharmacology and potentially response to serotonergic substances.4
Of Sequences and Psychedelics
At least seven nonsynonymous SNPs have been found within the coding region of the HTR2A gene, located on chromosome 13. Interestingly, all seven SNPs comprise some of the most common genetic variants found in the population at large.5 The authors comment that the exact locations of these polymorphisms, in specific regions of the gene that are critical for both structure and function of the receptor, likely impact pharmacological response; they map to critical regions like the N and C terminal ends, both of which are important in signaling and regulatory functions, and various “loops” of the receptor that recognize and bind specific ligands.3,6
This research was performed in vitro, in which cells expressing 5-HT2ARs were grown and plated, and their biological responses to the application of substances (or serotonin) was recorded. The response to treatment, measured by the interactions between proteins within the cells, was drug-specific; no single SNP variant induced a uniform effect across all substances tested (psilocin, mescaline, 5-MeO-DMT, and LSD). The authors visualized intracellular events and protein interactions in cells harboring each variant 5-HT2AR using a biofluorescence technique, allowing proteins and their crucial components to be tagged with a fluorescent insert and their activity within the cell to be tracked. The alpha subunit of the GPCR, Gαq, was tagged to allow for surveillance of the downstream effects that occur following receptor activation. While none of the variants tested showed standard Wild Type (WT) 5-HT2AR responses to the application of serotonin, some polymorphic receptors exhibited significant, albeit modest, drug-specific changes in potency of response (Figure 1).

Figure 1: Average Gαq dissociation concentration-response curves for each drug tested. A) psilocin, B) 5-MeO-DMT, C) mescaline, and D) LSD at Wild-Type (WT) versus polymorphic 5-HT2A receptors using a biofluorescence tracking technique. Data was fitted to a standardized scale, where 0% is the baseline signal and 100% is the maximum serotonin-stimulated signal for the WT receptor (along the Y axis). For each drug-receptor pair, data from at least three independent experiments were used, with the EC50 (the half-maximal effective concentration, being the concentration of the drug needed to cause half the maximum effect) averaged between each trial. From Schmitz et al., 2022.
The activity of β-arrestin (βArr) proteins, known to regulate GPCR function at various levels,7 was also probed. These proteins are known to colocalize with 5-HT2A receptors, and likely play regulatory roles by altering the interactions between the subunits of the receptor and impacting the cell’s response by affecting receptor expression, among other biologically significant functions.8,9 When activating the 5-HT2AR, some substances mediate their activity through preferential activation of Gαq or βArr signaling, known as biased signaling. LSD exerts its effects via βArr2, downstream of the 5-HT2AR, for example, and mice for which βArr2 has been silenced or removed (“knock-out mice”) exhibit a markedly diminished or altogether absent response to LSD.10,11 The intensity of βArr2 signaling was increased in response to 5-MeO-DMT, mescaline, and LSD as compared to WT controls, with the Ala230Thr variant exhibiting the most significant changes (Figure 2).

Figure 2: Average concentration-response curves for βArr1 recruitment in A) psilocin, B) 5-MeO-DMT, C) mescaline, and D) LSD, and βArr2 recruitment for E) psilocin, F) 5-MeO-DMT, G) mescaline, and H) LSD at WT versus polymorphic Ala230Thr 5-HT2ARs using βArr1 and βArr2 biofluorescence assays. In both assays, the data recorded was fitted to a standardized scale, with 0% being the baseline response and 100% being the maximum response at the WT receptor upon serotonin stimulation (along the Y axis). Again, for each drug-receptor pair, data from at least three independent trials was used and averaged. Significant differences in EC50 as compared to WT were found in the βArr2 recruitment assay for 5-MeO-DMT, mescaline, and LSD. From Schmitz et al., 2022.
Altered States, Altered Rates
Changes in receptor function over time in response to various agonists, in addition to changes in ligand-specific biases, have been previously studied.12 βArr2 signaling in response to LSD has been shown to increase in potency of response, increasing LSD’s efficacy over time, which may be facilitated by its lengthy time of residence at the 5-HT2AR.13 The signaling and kinetics for the binding and activity of psilocin, 5-MeO-DMT, and mescaline have not been well characterized until now.
Utilizing the same biofluorescence assay, researchers measured both Gαq and βArr2 activity in vitro in WT receptors at 5 minutes, 30 minutes, 1 hour, 2 hours, and 3 hours. For psilocin, a significant increase in Gαq-mediated signaling and a decrease in βArr2 signaling was observed (showing a bias towards Gαq signaling over time); a similar increase in Gαq pathways over time was found for 5-MeO-DMT, but no appreciable change in βArr2, again exhibiting a Gαq bias over time. LSD showed the greatest change in Gαq and βArr2 potency over time (550-fold and 200-fold after 2 hours, respectively) (Figure 3). These profound changes again reflect LSD’s long duration at the 5-HT2AR, allowing for a dramatic change in bias over time. Serotonin itself showed only a comparatively slight increase in Gαq and reduction in βArr2 recruitment over time.
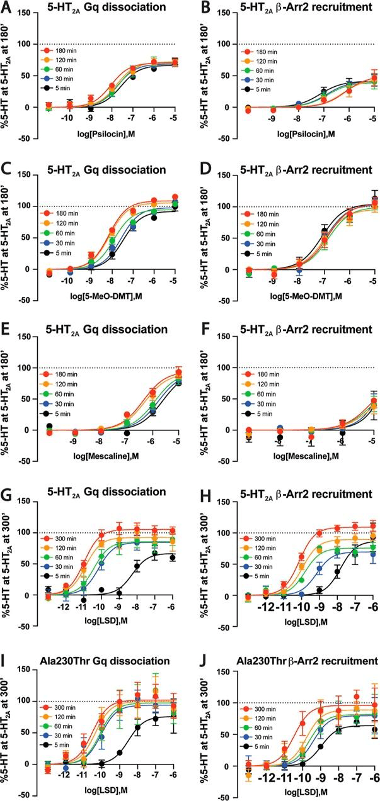
Figure 3: The rate for 5-HT2AR Gαq dissociation for A) psilocin, C) 5-MeO-DMT, E) mescaline, and G) LSD at WT versus I) polymorphic (Ala230Thr) 5-HT2ARs. The rate for 5-HT2AR βArr2 recruitment for B) psilocin, D) 5-MeO-DMT, F) mescaline, H) LSD at WT versus J) polymorphic (Ala230Thr) 5-HT2ARs. Data were normalized according to the same scale, with 0% being baseline and 100% being the maximal response to serotonin stimulation at the WT receptor (along the Y axis). As in the previous data, three independent trials were performed and the EC50s averaged. From Schmitz et al., 2022.
Because LSD induced the most profound changes in signaling kinetics, its activity was further studied at both WT and polymorphic receptors over 5 hours’ time. Echoing the findings of the previous Gαq assay, no changes in Gαq signaling between WT and variant receptors was evident, at five minutes nor any time over the five hour observation period. Regarding βArr2 recruitment, the Ala230Thr variant of the 5-HT2AR was the only one in which significant increases were found compared to the WT receptor at 5 minutes. Compared to the WT receptor, none of the variant receptors differed in βArr2 potency for LSD at 30 minutes or 1 hour, while the His452Tyr variant was associated with significant increases compared to WT at 2 and 5 hours. This also reflects the findings of the previous assays for βArr2 activity, where the Ala230Thr variant was associated with a near-immediate and appreciable change in activity and the His452Tyr variant following, both demonstrating altered profiles in response to LSD application and favoring Gαq signaling to βArr2 over time. The bias towards Gαq signaling in these two variants differs from the WT 5HT2AR’s preference for βArr2 activity in response to LSD.
Expression and Detection of Variant 5-HT2A Receptors
To test if 5-HT2AR density was affected by SNPs, the authors measured the total quantity of receptors (called the Bmax value) expressed on the surface of the cells being studied. Radioactive ketanserin, a 5-HT2A blocker, was used and a radioligand binding assay to track its activity. No uniform changes in receptor density or ligand potency were found, even though both the Ser12Asn and Ala230Thr variants expressed 5-HT2ARs at densities greater than WT. This led the authors to conclude that the changes in pharmacological activity and kinetics across the seven variants studied are likely not attributable to the differential expression of receptors, and more likely due to changes in the structure and function of the 5-HT2AR due to SNPs.
Conclusion
As several psychedelic drugs have entered clinical trials, the finding that various nonsynonymous SNPs can alter the pharmacology of these substances is significant, as future research can be designed and interpreted considering this fact. That changes in a single amino acid, while the HT2RA gene calls for 471 amino acids in total,14 is telling of the intricate structure of these receptors and the impact that even small changes in the genetic code can have on a larger scale. While some of the specific variants referenced here have been studied in the context of response to antipsychotic drugs,15,16 these results may be generalized to other 5-HT2A-acting substances like psychedelics.
The authors note that future research should address whether the observed changes in pharmacology are replicated in the serotonergic neurons of animal models and humans, in addition to the in vitro transfected cells studied here. They also mention that translational activity, the making of proteins from a genetic transcript, should be probed in these variants as deviations in the genetic code can demonstrably alter both the activity and expression of 5-HT2ARs. Importantly, these results suggest that certain individuals or groups expressing respective 5-HT2AR polymorphisms may respond differently to psychedelic-assisted therapy. The idiosyncratic, drug-specific responses observed throughout the study may also be implicated in these differences. This conclusion is particularly important as the action and effects of psychedelics continues to be studied in the context of therapeutic responses.
